Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 113QRT
Related questions
Question
100%
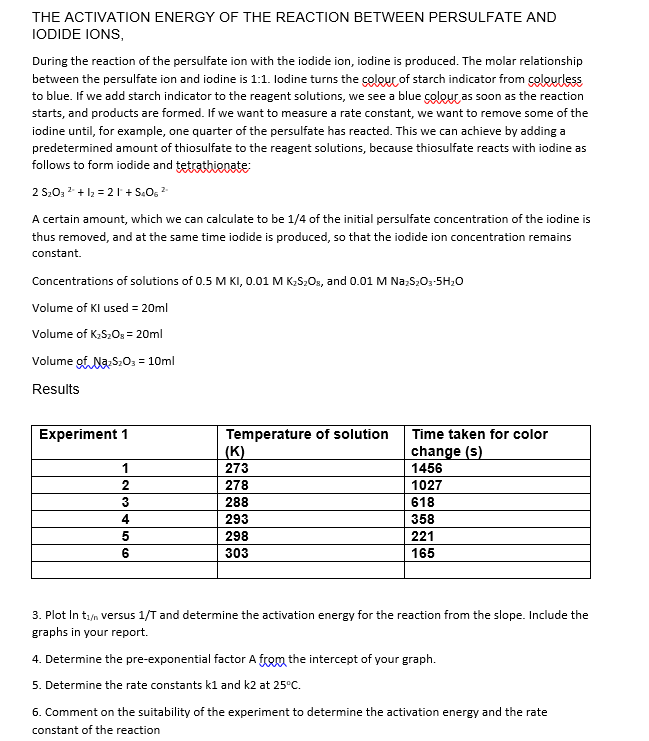
Transcribed Image Text:THE ACTIVATION ENERGY OF THE REACTION BETWEEN PERSULFATE AND
IODIDE IONS,
During the reaction of the persulfate ion with the iodide ion, iodine is produced. The molar relationship
between the persulfate ion and iodine is 1:1. Iodine turns the colour of starch indicator from colourless
to blue. If we add starch indicator to the reagent solutions, we see a blue colour as soon as the reaction
starts, and products are formed. If we want to measure a rate constant, we want to remove some of the
iodine until, for example, one quarter of the persulfate has reacted. This we can achieve by adding a
predetermined amount of thiosulfate to the reagent solutions, because thiosulfate reacts with iodine as
follows to form iodide and tetrathionate:
2 S₂03² + 1₂ = 21 +S406 ²
A certain amount, which we can calculate to be 1/4 of the initial persulfate concentration of the iodine is
thus removed, and at the same time iodide is produced, so that the iodide ion concentration remains
constant.
Concentrations of solutions of 0.5 M KI, 0.01 M K₂S₂Os, and 0.01 M Na₂S₂O3-5H₂O
Volume of KI used = 20ml
Volume of K₂S₂O = 20ml
Volume of Na2S₂O3 = 10ml
Results
Experiment 1
1
2
3
4
5
6
Temperature of solution
(K)
273
278
288
293
298
303
Time taken for color
change (s)
1456
1027
618
358
221
165
3. Plot In t₁/n versus 1/T and determine the activation energy for the reaction from the slope. Include the
graphs in your report.
4. Determine the pre-exponential factor A from the intercept of your graph.
5. Determine the rate constants k1 and k2 at 25°C.
6. Comment on the suitability of the experiment to determine the activation energy and the rate
constant of the reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
4. Determine the pre-exponential factor A from the intercept of your graph.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning