Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.26P
Related questions
Question
question 4
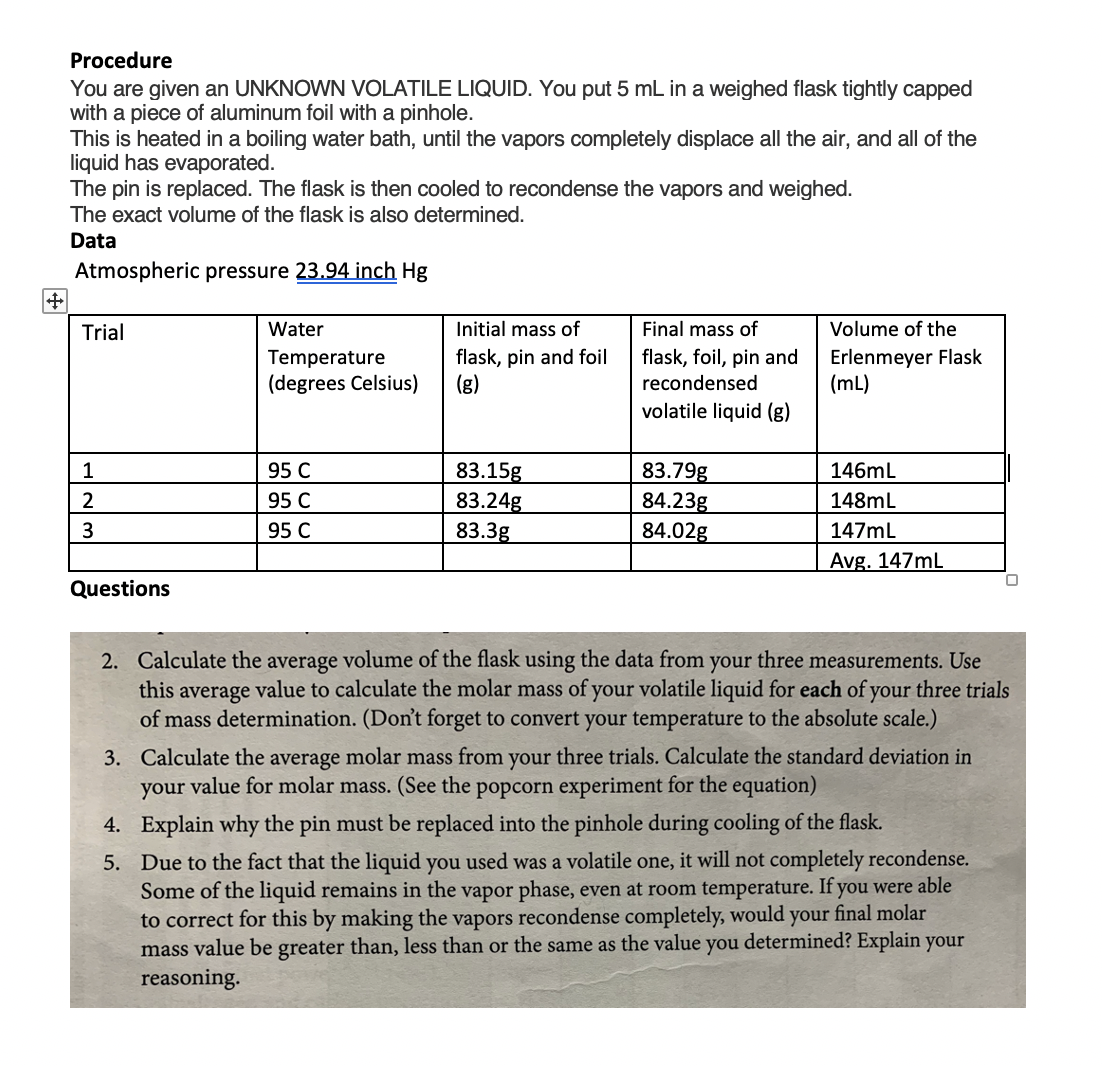
Transcribed Image Text:Procedure
You are given an UNKNOWN VOLATILE LIQUID. You put 5 mL in a weighed flask tightly capped
with a piece of aluminum foil with a pinhole.
This is heated in a boiling water bath, until the vapors completely displace all the air, and all of the
liquid has evaporated.
The pin is replaced. The flask is then cooled to recondense the vapors and weighed.
The exact volume of the flask is also determined.
Data
Atmospheric pressure 23.94 inch Hg
Trial
Water
Initial mass of
Final mass of
Volume of the
Temperature
(degrees Celsius)
flask, pin and foil
(g)
flask, foil, pin and
recondensed
Erlenmeyer Flask
(mL)
volatile liquid (g)
83.79g
84.23g
84.02g
95 C
83.15g
83.24g
83.3g
1
146mL
2
95 C
148mL
3
95 C
147mL
Avg. 147mL
Questions
2. Calculate the average volume of the flask using the data from your three measurements. Use
this average value to calculate the molar mass of your volatile liquid for each of your three trials
of mass determination. (Don't forget to convert your temperature to the absolute scale.)
3. Calculate the average molar mass from your three trials. Calculate the standard deviation in
your value for molar mass. (See the popcorn experiment for the equation)
4. Explain why the pin must be replaced into the pinhole during cooling of the flask.
5. Due to the fact that the liquid you used was a volatile one, it will not completely recondense.
Some of the liquid remains in the vapor phase, even at room temperature. If you were able
to correct for this by making the vapors recondense completely, would your final molar
mass value be greater than, less than or the same as the value you determined? Explain your
reasoning.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Due to the fact that the liquid you used was a volatile one, it will not completely recondense. Some of the liquid remains in the vapor phase, even at room temperature. If you were able to correct for this by making the vapors recondense completely, would your final molar mass value be greater than, less than or the same as the value you determined? Explain your reasoning.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning