Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
4
Transcribed Image Text:A-. A-
Nmal
No Spacing
Styles
Pare
Dictate
Sensitity
Editor
Open
Grammarly
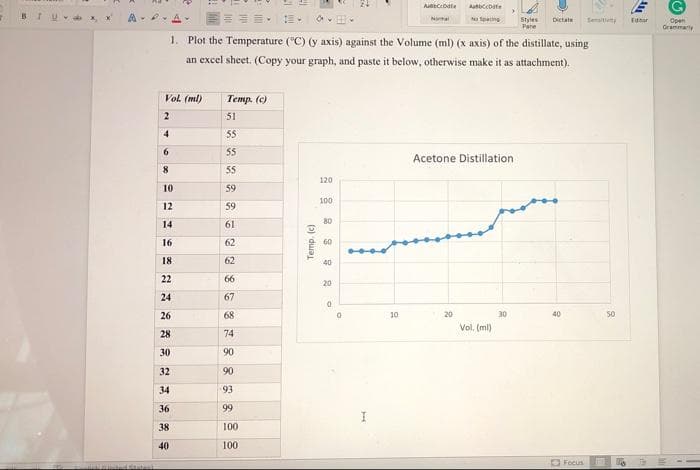
1. Plot the Temperature ("C) (y axis) against the Volume (ml) (x axis) of the distillate, using
an excel sheet. (Copy your graph, and paste it below, otherwise make it as attachment).
Vol. (ml)
Temp. (c)
2
51
55
6.
55
Acetone Distillation
55
120
10
59
100
12
59
80
14
61
16
62
60
18
62
40
22
66
20
24
67
26
68
10
20
30
40
50
Vol. (ml)
28
74
30
90
32
90
34
93
36
99
38
100
40
100
O Focus
Temp. (c)
Transcribed Image Text:Grammerty
Clou
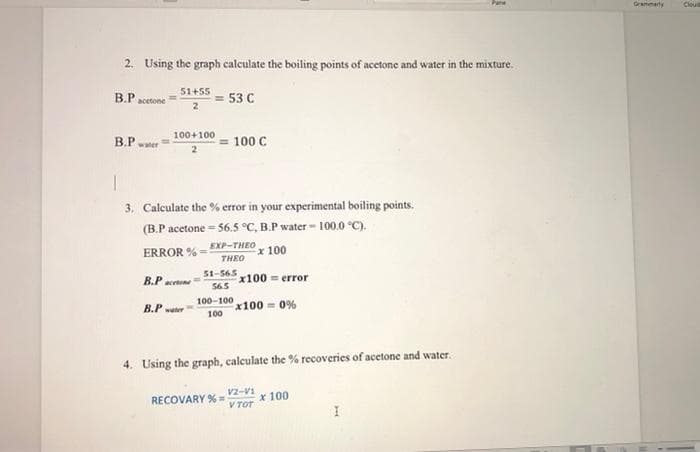
2. Using the graph calculate the boiling points of acetone and water in the mixture.
51+55
B.P acetone
= 53 C
100+100
B.P waer
100 C
3. Calculate the % error in your experimental boiling points.
(B.P acetone = 56.5 °C, B.P water - 100.0 °C).
EXP-THEO
ERROR % =
x 100
THEO
51-565
x100 error
565
В.Р
acetone
100-100
x100 = 0%
100
B.P ter
4. Using the graph, calculate the % recoveries of acetone and water.
V2-V1
x 100
V TOT
RECOVARY % =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
