4. Using the phase diagram to the right: wod. abom en Label each phase in the diagram to the right in the box provided. a. b. What is the normal melting and normal boiling points? normal melting 54.8 Kelvin Point. everi izum achter to amoi wod noite normal boiling point 290.2 kelvin e. 49.8 f. P atm 1.00 Solid A. 0.00150 ya baapeist 910 foerto L Sbeaulbero need liquid B C. What is the phase change associated with letter A when the pressure is suddenly dropped? Gras phase sche lo ambig ynom word toen eipeneg of bezu onied arow noilope sirit 11 ejutoteqmel erti seos of food rouons sipiensp of botopered of evor bluow bed id. What singular change can you do to letter B to vaporize it? to poss (tal ogrodo airil tol bebeen ygiene ori brit bno.Oºp\LASEN What is happening on the molecular level for your answer in part D to work? Gas 54.4 54.8 What is the vapor pressure of this substance at approximately 80K? T Kelvin 90.2 154
4. Using the phase diagram to the right: wod. abom en Label each phase in the diagram to the right in the box provided. a. b. What is the normal melting and normal boiling points? normal melting 54.8 Kelvin Point. everi izum achter to amoi wod noite normal boiling point 290.2 kelvin e. 49.8 f. P atm 1.00 Solid A. 0.00150 ya baapeist 910 foerto L Sbeaulbero need liquid B C. What is the phase change associated with letter A when the pressure is suddenly dropped? Gras phase sche lo ambig ynom word toen eipeneg of bezu onied arow noilope sirit 11 ejutoteqmel erti seos of food rouons sipiensp of botopered of evor bluow bed id. What singular change can you do to letter B to vaporize it? to poss (tal ogrodo airil tol bebeen ygiene ori brit bno.Oºp\LASEN What is happening on the molecular level for your answer in part D to work? Gas 54.4 54.8 What is the vapor pressure of this substance at approximately 80K? T Kelvin 90.2 154
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 38GQ: The following data are the equilibrium vapor pressure of limonene, C10H16, at various temperatures....
Related questions
Question
Ch. 9&10 Please help with question 4 (d-f)
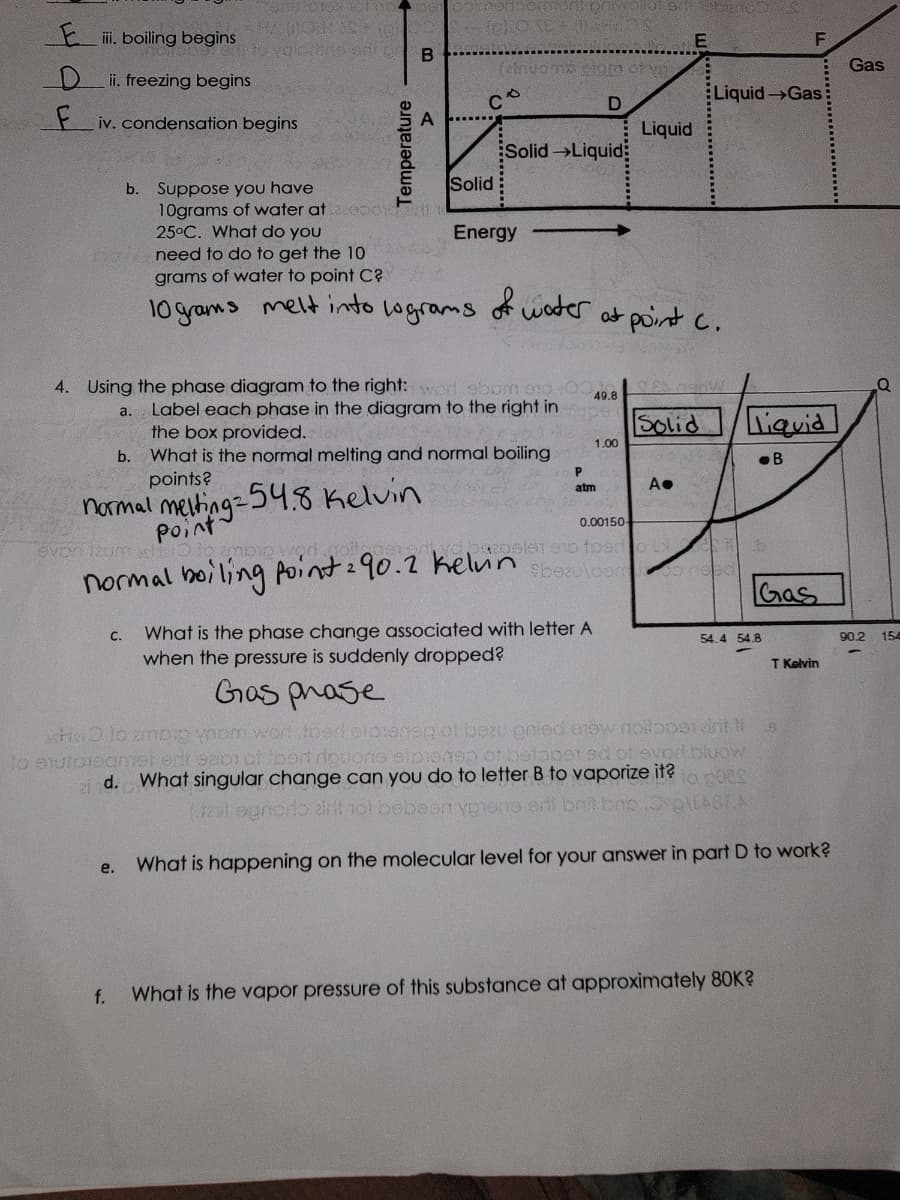
Transcribed Image Text:E. boiling begins
ii. freezing begins
F iv. condensation begins
7568/2010 10100
b. Suppose you have
10grams of water at
25°C. What do you
a.
b.
e.
f.
Temperature
need to do to get the 10
grams of water to point C?
melt into
10 grams
C.
normal melting = 54.8 Kelvin
Point
point
everlicum achter to ampio wad goitger
B
4
lograms
4. Using the phase diagram to the right: worl abom en
Label each phase in the diagram to the right in
the box provided.
What is the normal melting and normal boiling
points?
prwilot pri
(oko TE GROS
E
Solid
(ainuomp glom of ve
D
Solid Liquid
Energy
normal boiling point 290.2 kelvin
of water at point C.
49.8
upe
1.00
Liquid
P
atm
Solid
A●
Liquid Gas
0.00150
sis1 910 foerto 6
Sbeaulbero mad
liquid
.B
What is the phase change associated with letter A
when the pressure is suddenly dropped?
Gas phase
schlo zmbip ynom word foed eipeneg of bezu pnied erow noilope arit 11
to ejutoteqmel erti saion of toert riquons etpienep of betoper ed of evor bluow
id. What singular change can you do to letter B to vaporize it? to poss
(talognoro airit tol bebeen ypione orit brit bno.Oºp\LASEA
What is happening on the molecular level for your answer in part D to work?
F
Gas
54.4 54.8
What is the vapor pressure of this substance at approximately 80K?
T Kelvin
5
Gas
90.2 154
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning