4. Which one of the following statements is INCORRECT? a. Polonium is the only metal that has a primitive cubic lattice. b. The lattice structure of the alkali metals is body-centered cubic. c. Many metals can crystallize in more than one type of crystal lattice. d. Metals with a body-centered cubic lattice contain a net of four metal atoms per unit cell. e. A hexagonal close packed structure is not an example of a cubic unit cell.
4. Which one of the following statements is INCORRECT? a. Polonium is the only metal that has a primitive cubic lattice. b. The lattice structure of the alkali metals is body-centered cubic. c. Many metals can crystallize in more than one type of crystal lattice. d. Metals with a body-centered cubic lattice contain a net of four metal atoms per unit cell. e. A hexagonal close packed structure is not an example of a cubic unit cell.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section12.1: Crystal Lattices And Unit Cells
Problem 1CYU: (a) Determining an Atom Radius from Lattice Dimensions: Gold has a face-centered unit cell, and its...
Related questions
Question
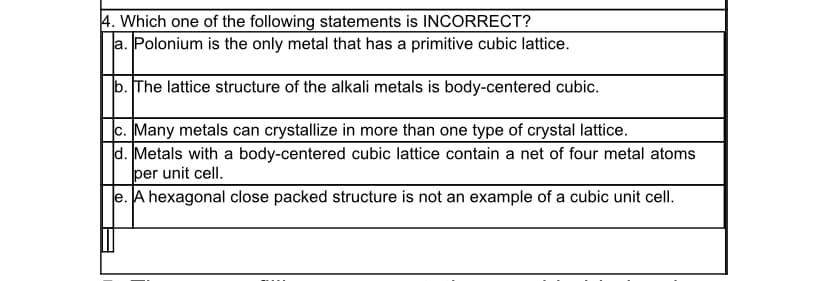
Transcribed Image Text:4. Which one of the following statements is INCORRECT?
a. Polonium is the only metal that has a primitive cubic lattice.
b. The lattice structure of the alkali metals is body-centered cubic.
c. Many metals can crystallize in more than one type of crystal lattice.
d. Metals with a body-centered cubic lattice contain a net of four metal atoms
per unit cell.
e. A hexagonal close packed structure is not an example of a cubic unit cell.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning