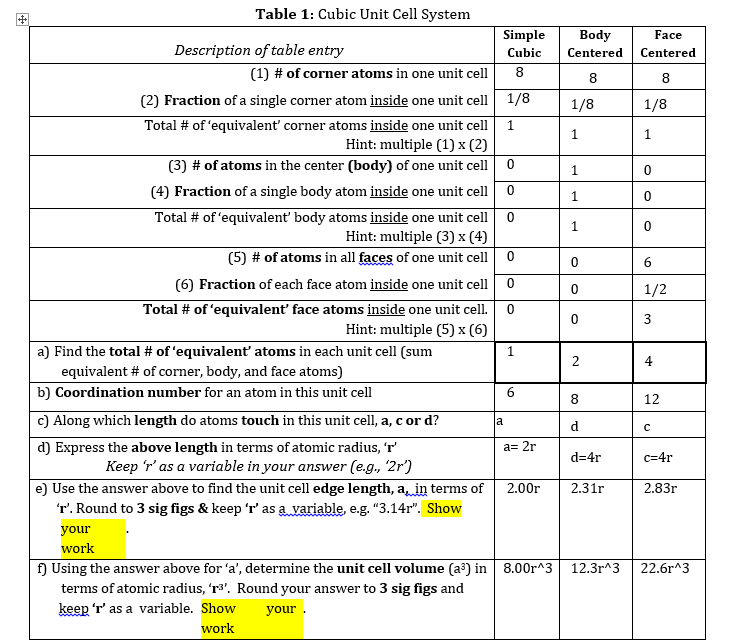
А. this question. Sketch one 'face' of a unit cell for SC, FCC and BCC. A total of 3 sketches are required for В. Calculate the percent empty space in each of the unit cells, SC, BCC and FCC. This is the ratio of space filled by atoms inside the unit cell to the total volume of the unit cell, given as a percentage. Remember that the volume of a sphere with radius, r, is ar³. (volume of unit cell – volume of atoms inside unit cell) volume of unit cell % Empty space x 100% a) Calculate the volume of ONE unit cell, keep the 'r' term in your result. b) Calculate the volume of all atoms inside ONE unit cell, again keep the 'r' term in your result. c) Use the equation above and your previous two answers to calculate the % empty space in the unit cell. You should find the r' terms cancel! Simplify and report your answer to 3 sig. figs. C. Density is an intensive property, meaning any sample size will give the same value. Hence, even a sample as small as a unit cell can be used to determine a substance's density: mass of atoms inside unit cell (in grams) density (using unit cell) = volume of the unit cell, a³ (in cm³) Using the density formula above, calculate the density (in g/cm³) for each of the three crystal structures (SC, FCC, & BCC) when they contain atoms with a mass of 105.00 amu and a radius of 0.125 nm. For each unit cell type, label and show how a calculation for the mass of the unit cell, the volume of the unit cell and the density of the unit cell. Show all necessary work, including units for each numeric value used in these calculations! HINT: Be careful with your unit conversions.if your density values are not between 0.5 and 30 g/cm³, you've likely made a unit conversion error. Which one of the three types of cubic unit cells (SC, BCC, FCC) corresponds to what is called "cubic closest packing"? Use two pieces of evidence from Table 1 and the previous questions to explain and support your selection. (just listing them is not an explanation and earns no points)
Basics in Organic Reactions Mechanisms
In organic chemistry, the mechanism of an organic reaction is defined as a complete step-by-step explanation of how a reaction of organic compounds happens. A completely detailed mechanism would relate the first structure of the reactants with the last structure of the products and would represent changes in structure and energy all through the reaction step.
Heterolytic Bond Breaking
Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. It is defined as breaking of a covalent bond between two different atoms in which one atom gains both of the shared pair of electrons. The atom that gains both electrons is more electronegative than the other atom in covalent bond. The energy needed for heterolytic fission is called as heterolytic bond dissociation energy.
Polar Aprotic Solvent
Solvents that are chemically polar in nature and are not capable of hydrogen bonding (implying that a hydrogen atom directly linked with an electronegative atom is not found) are referred to as polar aprotic solvents. Some commonly used polar aprotic solvents are acetone, DMF, acetonitrile, DMSO, etc.
Oxygen Nucleophiles
Oxygen being an electron rich species with a lone pair electron, can act as a good nucleophile. Typically, oxygen nucleophiles can be found in these compounds- water, hydroxides and alcohols.
Carbon Nucleophiles
We are aware that carbon belongs to group IV and hence does not possess any lone pair of electrons. Implying that neutral carbon is not a nucleophile then how is carbon going to be nucleophilic? The answer to this is that when a carbon atom is attached to a metal (can be seen in the case of organometallic compounds), the metal atom develops a partial positive charge and carbon develops a partial negative charge, hence making carbon nucleophilic.
pls help q(A) and (D)

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 6 images









