Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter2: Alkanes And Cycloalkanes
Section: Chapter Questions
Problem 2.49P: Use your answers from Problem 2.48 to complete the table showing correlations between cis,trans and...
Related questions
Question
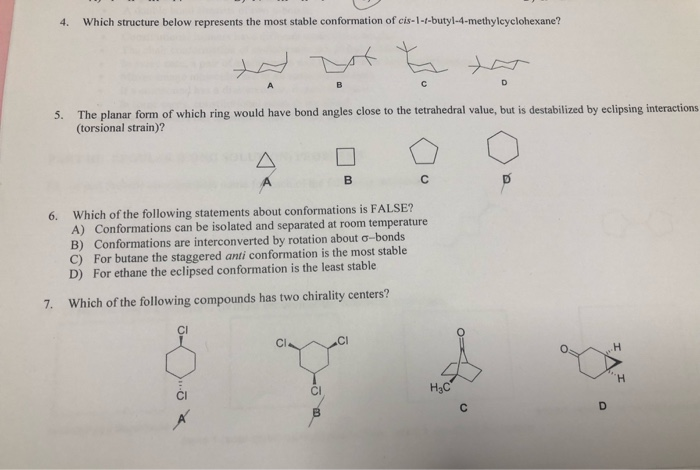
Transcribed Image Text:4.
Which structure below represents the most stable conformation of cis-1-t-butyl-4-methylcyclohexane?
A
B
D
5.
The planar form of which ring would have bond angles close to the tetrahedral value, but is destabilized by eclipsing interactions
(torsional strain)?
Which of the following statements about conformations is FALSE?
A) Conformations can be isolated and separated at room temperature
B) Conformations are interconverted by rotation about o-bonds
C) For butane the staggered anti conformation is the most stable
D) For ethane the eclipsed conformation is the least stable
6.
7.
Which of the following compounds has two chirality centers?
.H
H.
H3C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
