4.38 Classify each of the following aqueous solutions as a non- electrolyte, weak electrolyte, or strong electrolyte: (a) PBC12, (b) N(CH.). (c) CSOH, (d) HS. (e) CrCla. (f) Ni(CH,COO)e
4.38 Classify each of the following aqueous solutions as a non- electrolyte, weak electrolyte, or strong electrolyte: (a) PBC12, (b) N(CH.). (c) CSOH, (d) HS. (e) CrCla. (f) Ni(CH,COO)e
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.25QP: Would you expect a precipitation reaction between an ionic compound that is an electrolyte and an...
Related questions
Question
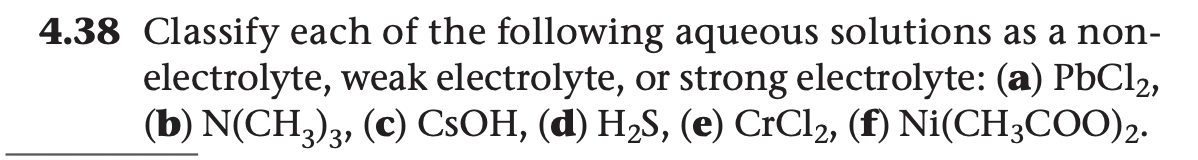
Transcribed Image Text:4.38 Classify each of the following aqueous solutions as a non-
electrolyte, weak electrolyte, or strong electrolyte: (a) PBC12,
(b) N(CH,), (c) CSOH, (d) H2S, (e) CrCl2, (f) Ni(CH;COO)2.
31
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning