40. How many moles of chloride ions are in 62.2 mL of a 2.00 M aluminum chloride solution? a. 0.124 mol b. 0.0415 mol c. 0.746 mol d. 0.373 mol e. 0.0311 mol
40. How many moles of chloride ions are in 62.2 mL of a 2.00 M aluminum chloride solution? a. 0.124 mol b. 0.0415 mol c. 0.746 mol d. 0.373 mol e. 0.0311 mol
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.63PAE: 3.63 How many moles of solute are present in each of these solutions? (a) 48.0 mL of 3.4 M H2SO4....
Related questions
Question
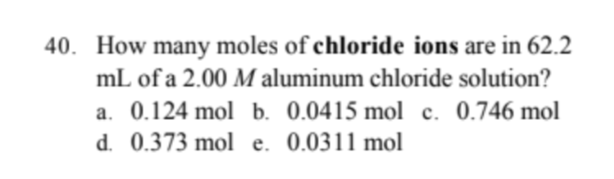
Transcribed Image Text:40. How many moles of chloride ions are in 62.2
mL of a 2.00 M aluminum chloride solution?
a. 0.124 mol b. 0.0415 mol c. 0.746 mol
d. 0.373 mol e. 0.0311 mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning