400 450 500 550 600 650 700 wavelength (nm) : Wavclength of maximum absorbance for base form ph shows that the basic form has a maximum absorption at Amax, while mum absorption at Amax. Explain why the basic form and acidic form ha ions at this specific wavelength. the importance of determining the Amax? ed Ka value for Bromocresol green is 1.6 x 10. Using your measured the percent error. laccepted-measured x 100 accepted calculate the pH of the solutions it is found by using the following equ (mol NaAc log al HAc
400 450 500 550 600 650 700 wavelength (nm) : Wavclength of maximum absorbance for base form ph shows that the basic form has a maximum absorption at Amax, while mum absorption at Amax. Explain why the basic form and acidic form ha ions at this specific wavelength. the importance of determining the Amax? ed Ka value for Bromocresol green is 1.6 x 10. Using your measured the percent error. laccepted-measured x 100 accepted calculate the pH of the solutions it is found by using the following equ (mol NaAc log al HAc
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.25P
Related questions
Question
100%
The measured value is from Graph is 0.915
Transcribed Image Text:mol HAC)
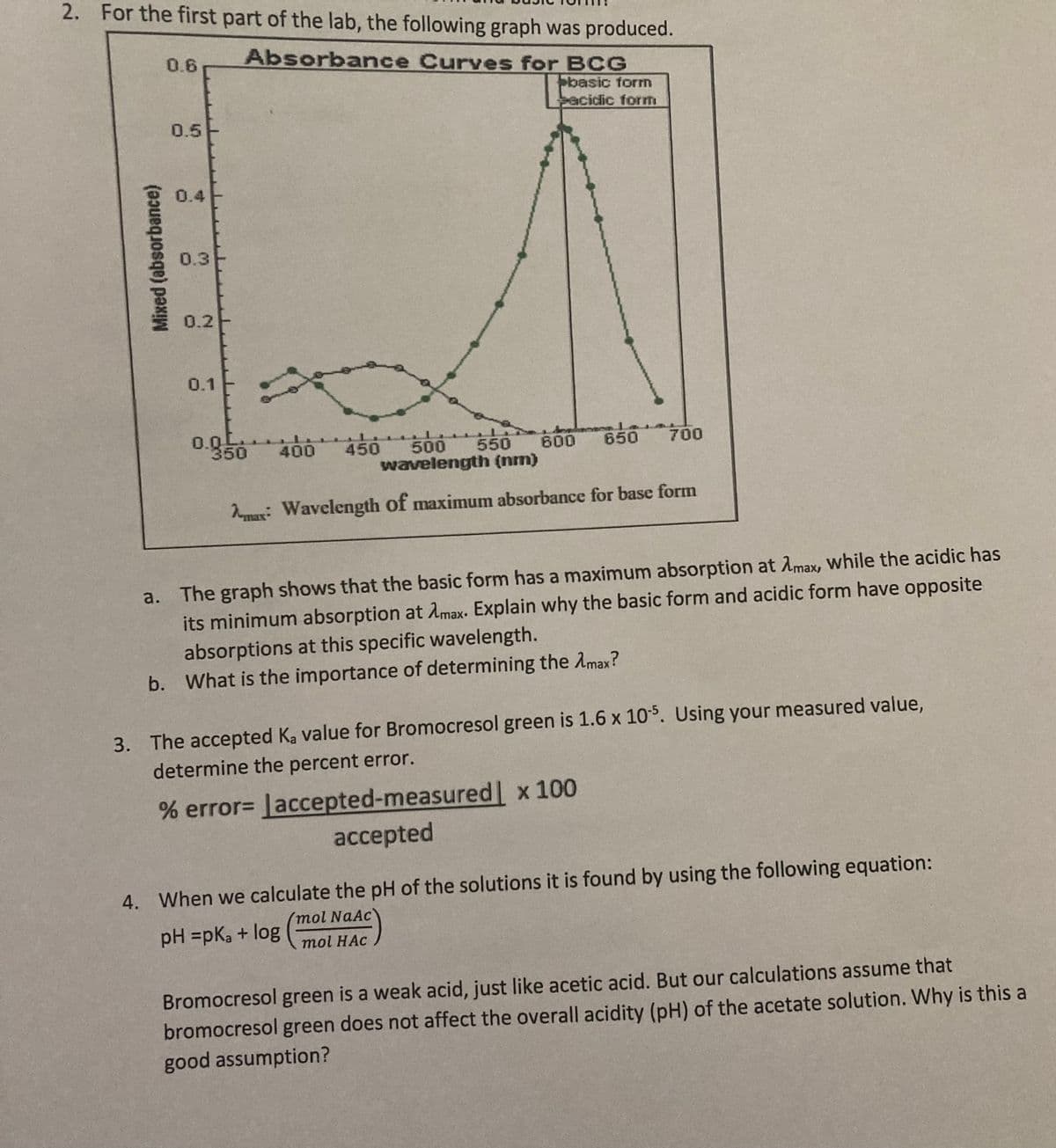
2. For the first part of the lab, the following graph was produced.
0.6
Absorbance Curves for BCG
basic forn
acidic form
0.5
0.4
0.3
0.2
0.1
0.0L
350
600 650
700
400
450
500
550
wavelength (nm)
Nmax: Wavelength of maximum absorbance for base form
a. The graph shows that the basic form has a maximum absorption at Amax, while the acidic has
its minimum absorption at Amax. Explain why the basic form and acidic form have opposite
absorptions at this specific wavelength.
b. What is the importance of determining the Amax?
3. The accepted Ka value for Bromocresol green is 1.6 x 105. Using your measured value,
determine the percent error.
% error= Jaccepted-measured x 100
accepted
4. When we calculate the pH of the solutions it is found by using the following equation:
(mol NaAc
pH =pK, + log
mol HAc
Bromocresol green is a weak acid, just like acetic acid. But our calculations assume that
bromocresol green does not affect the overall acidity (pH) of the acetate solution. Why is this a
good assumption?
Mixed (absorbance)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning