42. Which of the following statements about alkanes, alkenes and alkynes is FALSE? A. Alkanes can be solids, liquids or gases. B. Alkenes are always gases in state at room temperature. C. Alkanes are more reactive to halogenation compared to alkenes. D. Alkynes exhibit the greatest electron density among hydrocarbons 43. When alkenes are oxidized initially, they form what types of compounds? A. Aldehydes B. Alcohols C. Alkynes D. Alkanes 44. Complete combustion of hydrocarbons leads to the formation of two products. These products are: A. Carbon and water vapour B. Carbon dioxide and water vapour C. Carbon dioxide and carbon D. Water vapour and Water droplets 45. Which among the alkanes below has the highest boiling point? A. n-hexane B. isohexane C. 2,2-dimethylbutane D. 3-methylpentane 46. Which of the following compounds will require UV light to promote halogenation? Choose all possible answers. A. carbon tetrachloride B. pent-1-yne C. isopentane D. propene 47. Which of the following compounds will result from the reaction between propene and HBr? A. 2-bromopropane 1-bromonronano
42. Which of the following statements about alkanes, alkenes and alkynes is FALSE? A. Alkanes can be solids, liquids or gases. B. Alkenes are always gases in state at room temperature. C. Alkanes are more reactive to halogenation compared to alkenes. D. Alkynes exhibit the greatest electron density among hydrocarbons 43. When alkenes are oxidized initially, they form what types of compounds? A. Aldehydes B. Alcohols C. Alkynes D. Alkanes 44. Complete combustion of hydrocarbons leads to the formation of two products. These products are: A. Carbon and water vapour B. Carbon dioxide and water vapour C. Carbon dioxide and carbon D. Water vapour and Water droplets 45. Which among the alkanes below has the highest boiling point? A. n-hexane B. isohexane C. 2,2-dimethylbutane D. 3-methylpentane 46. Which of the following compounds will require UV light to promote halogenation? Choose all possible answers. A. carbon tetrachloride B. pent-1-yne C. isopentane D. propene 47. Which of the following compounds will result from the reaction between propene and HBr? A. 2-bromopropane 1-bromonronano
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter22: Organic And Biological Molecules
Section: Chapter Questions
Problem 4RQ: Summarize the nomenclature rules for alkanes, alkenes, alkynes, and aromatic compounds. Correct the...
Related questions
Question
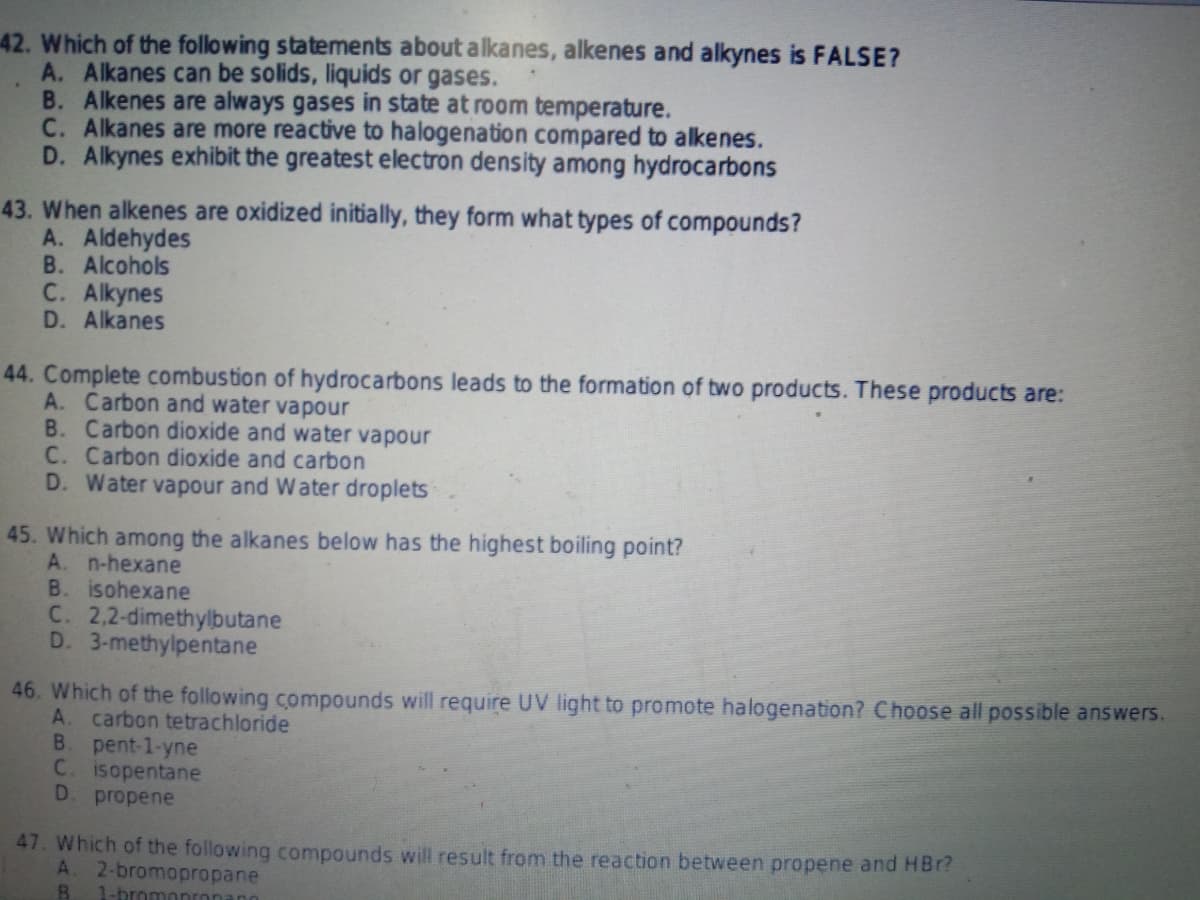
Transcribed Image Text:42. Which of the following statements about alkanes, alkenes and alkynes is FALSE?
A. Alkanes can be solids, liquids or gases.
B. Alkenes are always gases in state at room temperature.
C. Alkanes are more reactive to halogenation compared to alkenes.
D. Alkynes exhibit the greatest electron density among hydrocarbons
43. When alkenes are oxidized initially, they form what types of compounds?
A. Aldehydes
B. Alcohols
C. Alkynes
D. Alkanes
44. Complete combustion of hydrocarbons leads to the formation of two products. These products are:
A. Carbon and water vapour
B. Carbon dioxide and water vapour
C. Carbon dioxide and carbon
D. Water vapour and Water droplets
45. Which among the alkanes below has the highest boiling point?
A. n-hexane
B. isohexane
C. 2,2-dimethylbutane
D. 3-methylpentane
46. Which of the following compounds will require UV light to promote halogenation? Choose all possible answers.
A. carbon tetrachloride
B. pent-1-yne
C. isopentane
D. propene
47. Which of the following compounds will result from the reaction between propene and HBr?
A. 2-bromopropane
1-bromonronano
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co