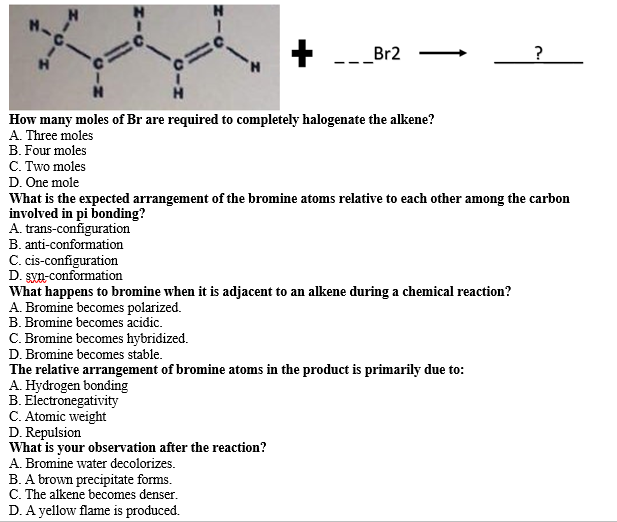
Br2 ? How many moles of Br are required to completely halogenate the alkene? A. Three moles B. Four moles C. Two moles D. One mole What is the expected arrangement of the bromine atoms relative to each other among the carbon involved in pi bonding? A. trans-configuration B. anti-conformation C. cis-configuration D. syn-conformation What happens to bromine when it is adjacent to an alkene during a chemical reaction? A. Bromine becomes polarized. B. Bromine becomes acidic. C. Bromine becomes hybridized. D. Bromine becomes stable. The relative arrangement of bromine atoms in the product is primarily due to: A. Hydrogen bonding B. Electronegativity C. Atomic weight D. Repulsion What is your observation after the reaction? A. Bromine water decolorizes. B. A brown precipitate forms. C. The alkene becomes denser. D. A yellow flame is produced.
Reactive Intermediates
In chemistry, reactive intermediates are termed as short-lived, highly reactive atoms with high energy. They rapidly transform into stable particles during a chemical reaction. In specific cases, by means of matrix isolation and at low-temperature reactive intermediates can be isolated.
Hydride Shift
A hydride shift is a rearrangement of a hydrogen atom in a carbocation that occurs to make the molecule more stable. In organic chemistry, rearrangement of the carbocation is very easily seen. This rearrangement can be because of the movement of a carbocation to attain stability in the compound. Such structural reorganization movement is called a shift within molecules. After the shifting of carbocation over the different carbon then they form structural isomers of the previous existing molecule.
Vinylic Carbocation
A carbocation where the positive charge is on the alkene carbon is known as the vinyl carbocation or vinyl cation. The empirical formula for vinyl cation is C2H3+. In the vinyl carbocation, the positive charge is on the carbon atom with the double bond therefore it is sp hybridized. It is known to be a part of various reactions, for example, electrophilic addition of alkynes and solvolysis as well. It plays the role of a reactive intermediate in these reactions.
Cycloheptatrienyl Cation
It is an aromatic carbocation having a general formula, [C7 H7]+. It is also known as the aromatic tropylium ion. Its name is derived from the molecule tropine, which is a seven membered carbon atom ring. Cycloheptatriene or tropylidene was first synthesized from tropine.
Stability of Vinyl Carbocation
Carbocations are positively charged carbon atoms. It is also known as a carbonium ion.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images




