45. What is the molecularity of the following elementary reaction? NH2CI(aq) + OH(aq) → NHCl(aq) + H2O(I) A. unimolecular bimolecular В. C. termolecular D. tetramolecular Е. Need to know the reaction order before molecularity can be determined. 46. Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. H* + H2O2 = H2O*-OH (rapid equilibrium) H2O*-OH + Br → HOBR + H20 (slow) HOBR + H* + Br – Br2 + H20 (fast) Which of the following rate laws is consistent with the mechanism? А. Rate = k[H2O2][H*]²[Br] Rate = k (H2O*-OH][Br] Rate = k[H2O2][H*][Br] Rate = k[HOB1][H*][Br][H2O2] Rate = k[Br В. %3D С. D. E.
45. What is the molecularity of the following elementary reaction? NH2CI(aq) + OH(aq) → NHCl(aq) + H2O(I) A. unimolecular bimolecular В. C. termolecular D. tetramolecular Е. Need to know the reaction order before molecularity can be determined. 46. Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. H* + H2O2 = H2O*-OH (rapid equilibrium) H2O*-OH + Br → HOBR + H20 (slow) HOBR + H* + Br – Br2 + H20 (fast) Which of the following rate laws is consistent with the mechanism? А. Rate = k[H2O2][H*]²[Br] Rate = k (H2O*-OH][Br] Rate = k[H2O2][H*][Br] Rate = k[HOB1][H*][Br][H2O2] Rate = k[Br В. %3D С. D. E.
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 60E: A possible mechanism for the decomposition of hydrogen peroxide is H2O22OHH2O2+OHH2O+HO2HO2+OHH2O+O2...
Related questions
Question
kindly answer the following question below, choose the best answer. thank u so much
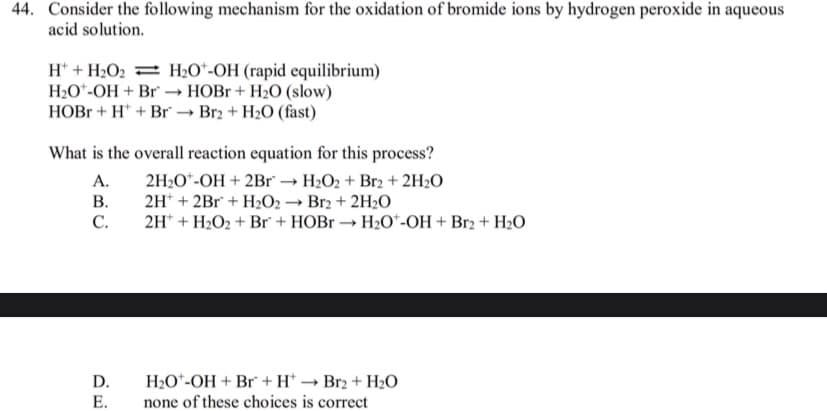
Transcribed Image Text:44. Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous
acid solution.
H* + H2O2 = H2O*-OH (rapid equilibrium)
H20*-OH + Br → HOB + H2O (slow)
HOB + H* + Br → Br2 + H2O (fast)
What is the overall reaction equation for this process?
A.
2H2O*-OH + 2Br → H2O2 + Br2 + 2H2O
В.
2H* + 2Br + H2O2 → Br2 + 2H2O
С.
2H* + H2O2 + Br + HOB → H2O*-OH + Br2 + H2O
D.
H20*-OH + Br + H* → Br2 + H2O
Е.
none of these choices is correct
![45. What is the molecularity of the following elementary reaction?
NH2CI(aq) + OH(aq) → NHCI(aq) + H20(I)
A.
unimolecular
bimolecular
В.
C.
termolecular
D.
tetramolecular
Е.
Need to know the reaction order before molecularity can be determined.
46. Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous
acid solution.
H* + H2O2 = H2O*-OH (rapid equilibrium)
H2O*-OH + Br" → HOB + H2O (slow)
HOB + H* + Br → Br2 + H2O (fast)
Which of the following rate laws is consistent with the mechanism?
А.
В.
Rate = k[H2O2][H*]°[Br]
Rate = k [H2O*-OH][Br]
Rate = k[H2O2][H*][Br]
Rate = k[HOBr][H*][Br][H2O2]
Rate = k[Br
%3D
С.
D.
E.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb231fffd-0090-498a-bbfa-fc9b5d8ef027%2F16be28fc-a5ff-4a0e-8a4c-190528767ea4%2Fmalc62sj_processed.jpeg&w=3840&q=75)
Transcribed Image Text:45. What is the molecularity of the following elementary reaction?
NH2CI(aq) + OH(aq) → NHCI(aq) + H20(I)
A.
unimolecular
bimolecular
В.
C.
termolecular
D.
tetramolecular
Е.
Need to know the reaction order before molecularity can be determined.
46. Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous
acid solution.
H* + H2O2 = H2O*-OH (rapid equilibrium)
H2O*-OH + Br" → HOB + H2O (slow)
HOB + H* + Br → Br2 + H2O (fast)
Which of the following rate laws is consistent with the mechanism?
А.
В.
Rate = k[H2O2][H*]°[Br]
Rate = k [H2O*-OH][Br]
Rate = k[H2O2][H*][Br]
Rate = k[HOBr][H*][Br][H2O2]
Rate = k[Br
%3D
С.
D.
E.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning