5) Acetylsalicylic acid (HC,H;O4) is a monoprotic acid commonly known as "aspirin". A typical aspirin tablet, however, contains only a small amount of the acid. In an experiment to determine its composition, an aspirin tablet was crushed and dissolved in water. It took 12.25 mL of 0.1466 M NaOH to neutralize the solution. Calculate the number of grains of aspirin in the tablet (one grain 0.0648 g). (assume acid:base ratio is 1:1).
5) Acetylsalicylic acid (HC,H;O4) is a monoprotic acid commonly known as "aspirin". A typical aspirin tablet, however, contains only a small amount of the acid. In an experiment to determine its composition, an aspirin tablet was crushed and dissolved in water. It took 12.25 mL of 0.1466 M NaOH to neutralize the solution. Calculate the number of grains of aspirin in the tablet (one grain 0.0648 g). (assume acid:base ratio is 1:1).
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter15: Complex Ion And Precipitation Equilibria
Section: Chapter Questions
Problem 57QAP: Calcium ions in blood trigger clotting. To prevent that in donated blood, sodium oxalate, Na2C2O4,...
Related questions
Question
May I get some help for # 5
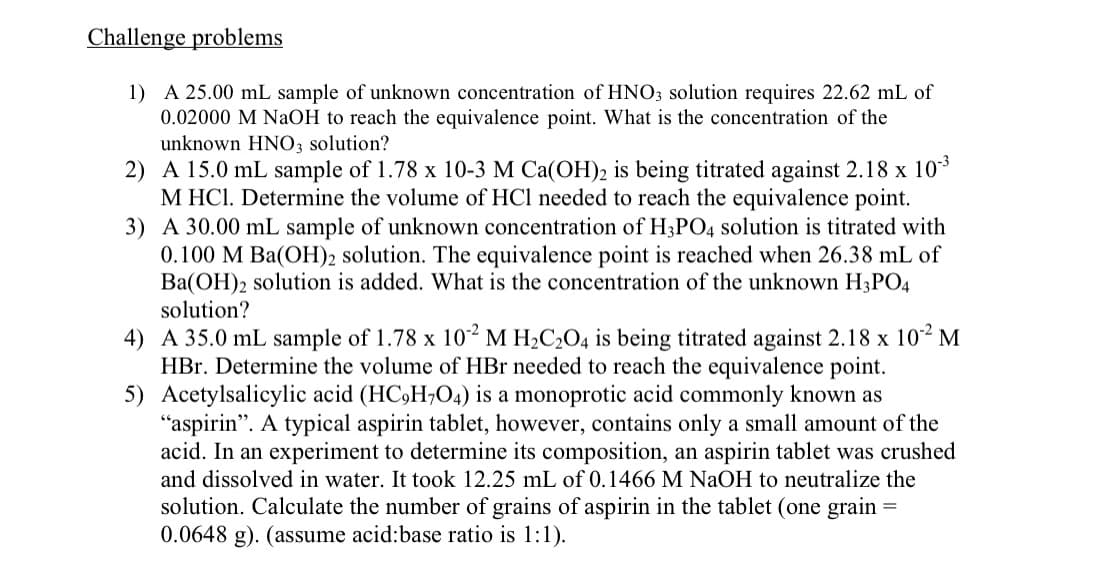
Transcribed Image Text:Challenge problems
1) A 25.00 mL sample of unknown concentration of HNO3 solution requires 22.62 mL of
0.02000 M NaOH to reach the equivalence point. What is the concentration of the
unknown HNO3 solution?
2) A 15.0 mL sample of 1.78 x 10-3 M Ca(OH)2 is being titrated against 2.18 x 10*
M HCl. Determine the volume of HCl needed to reach the equivalence point.
3) A 30.00 mL sample of unknown concentration of H3PO4 solution is titrated with
0.100 M Ba(OH)2 solution. The equivalence point is reached when 26.38 mL of
Ba(OH)2 solution is added. What is the concentration of the unknown H3PO4
solution?
4) A 35.0 mL sample of 1.78 x 10² M H2C2O4 is being titrated against 2.18 x 102 M
HBr. Determine the volume of HBr needed to reach the equivalence point.
5) Acetylsalicylic acid (HC,H;O4) is a monoprotic acid commonly known as
"aspirin". A typical aspirin tablet, however, contains only a small amount of the
acid. In an experiment to determine its composition, an aspirin tablet was crushed
and dissolved in water. It took 12.25 mL of 0.1466 M NaOH to neutralize the
solution. Calculate the number of grains of aspirin in the tablet (one grain
0.0648 g). (assume acid:base ratio is 1:1).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning