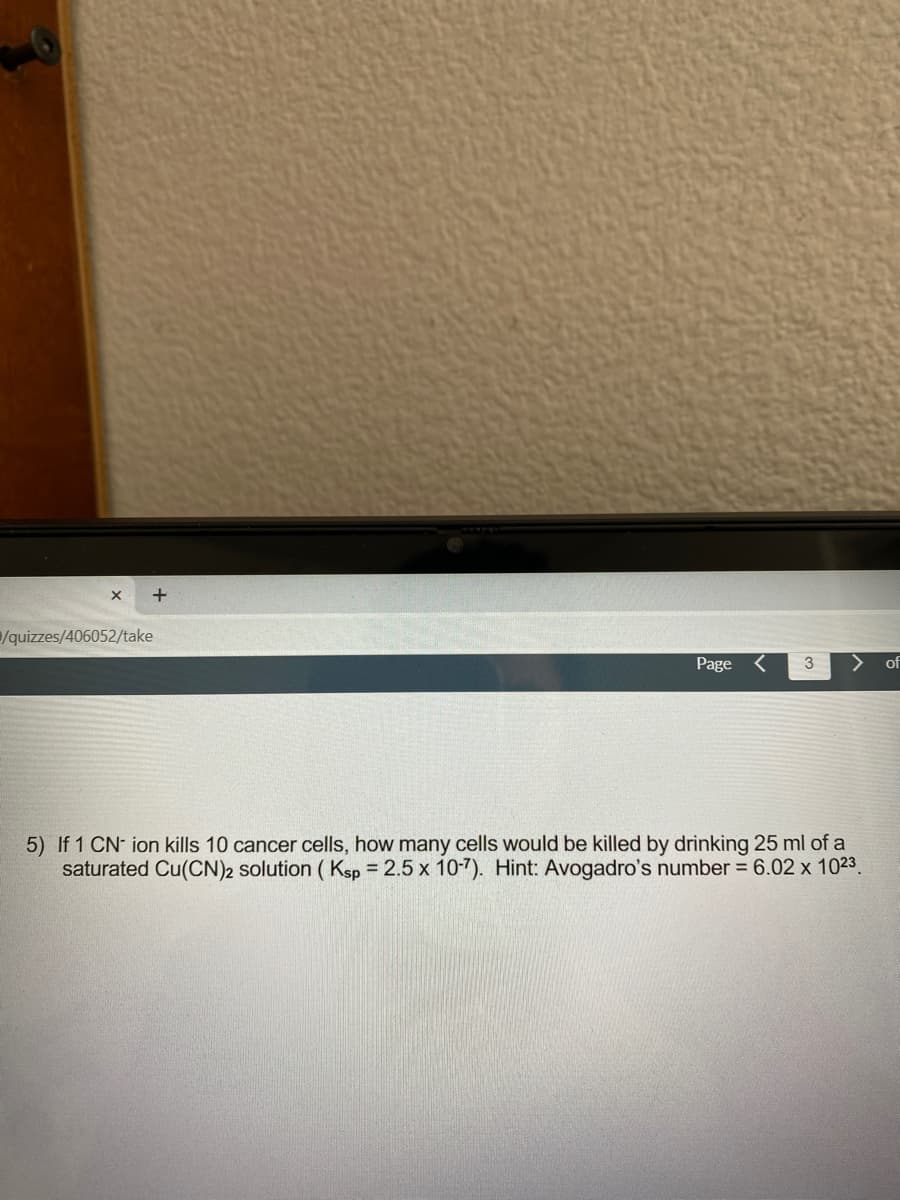
5) If 1 CN- ion kills 10 cancer cells, how many cells would be killed by drinking 25 ml of a saturated Cu(CN)2 solution ( Ksp = 2.5 x 10-7). Hint: Avogadro's number = 6.02 x 1023. %3D
5) If 1 CN- ion kills 10 cancer cells, how many cells would be killed by drinking 25 ml of a saturated Cu(CN)2 solution ( Ksp = 2.5 x 10-7). Hint: Avogadro's number = 6.02 x 1023. %3D
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 41PS
Related questions
Question
Transcribed Image Text:/quizzes/406052/take
Page
>
of
5) If 1 CN- ion kills 10 cancer cells, how many cells would be killed by drinking 25 ml of a
saturated Cu(CN)2 solution ( Ksp = 2.5 x 10-7). Hint: Avogadro's number = 6.02 x 1023.
Expert Solution
Step 1
Solubility product is expressed for sparingly soluble salt and Ksp is product of concentration of species in solution .
The question can be tackled by calculating no of moles from Ksp after calculating molarity that is solubility hence can be calculated the no of particles and then total cancer cells that can be killed.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning