5) Reaction order Enter the order of the reaction in bleach and the order in dye. Although the orders are integers, do not round your answers here; enter the numbers from your calculations without rounding in this step. Click on "Extra Info" for hint on what you are looking for in your data. order in bleach 2.311 Report to 3 significant figures order in dye 5.05 Report to 3 significant figures Evaluate Extra Info Correct! 6) Rate constants Round your exponents to integer values, and then use them to calculate the value of the rate constant for each trial. The units for k will vary depending on the overall order of the reaction. Enter the number only, but put that number in terms of the units appropriate for your overall experimentally-determined reaction order: (Units for different reaction orders are in "Extra Info" below.) k -1.062e23 Report to 3 significant figures k -1.062e23 Report to 3 significant figures k -1.062e23 Report to 3 significant figures units other units Evaluate Extra Info
5) Reaction order Enter the order of the reaction in bleach and the order in dye. Although the orders are integers, do not round your answers here; enter the numbers from your calculations without rounding in this step. Click on "Extra Info" for hint on what you are looking for in your data. order in bleach 2.311 Report to 3 significant figures order in dye 5.05 Report to 3 significant figures Evaluate Extra Info Correct! 6) Rate constants Round your exponents to integer values, and then use them to calculate the value of the rate constant for each trial. The units for k will vary depending on the overall order of the reaction. Enter the number only, but put that number in terms of the units appropriate for your overall experimentally-determined reaction order: (Units for different reaction orders are in "Extra Info" below.) k -1.062e23 Report to 3 significant figures k -1.062e23 Report to 3 significant figures k -1.062e23 Report to 3 significant figures units other units Evaluate Extra Info
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section11.1: Reaction Rate
Problem 11.2CE: Instantaneous rates for the reaction of hydroxide ion with Cv+ can be determined from the slope of...
Related questions
Question
Chemistry
How can I find the rate orders.
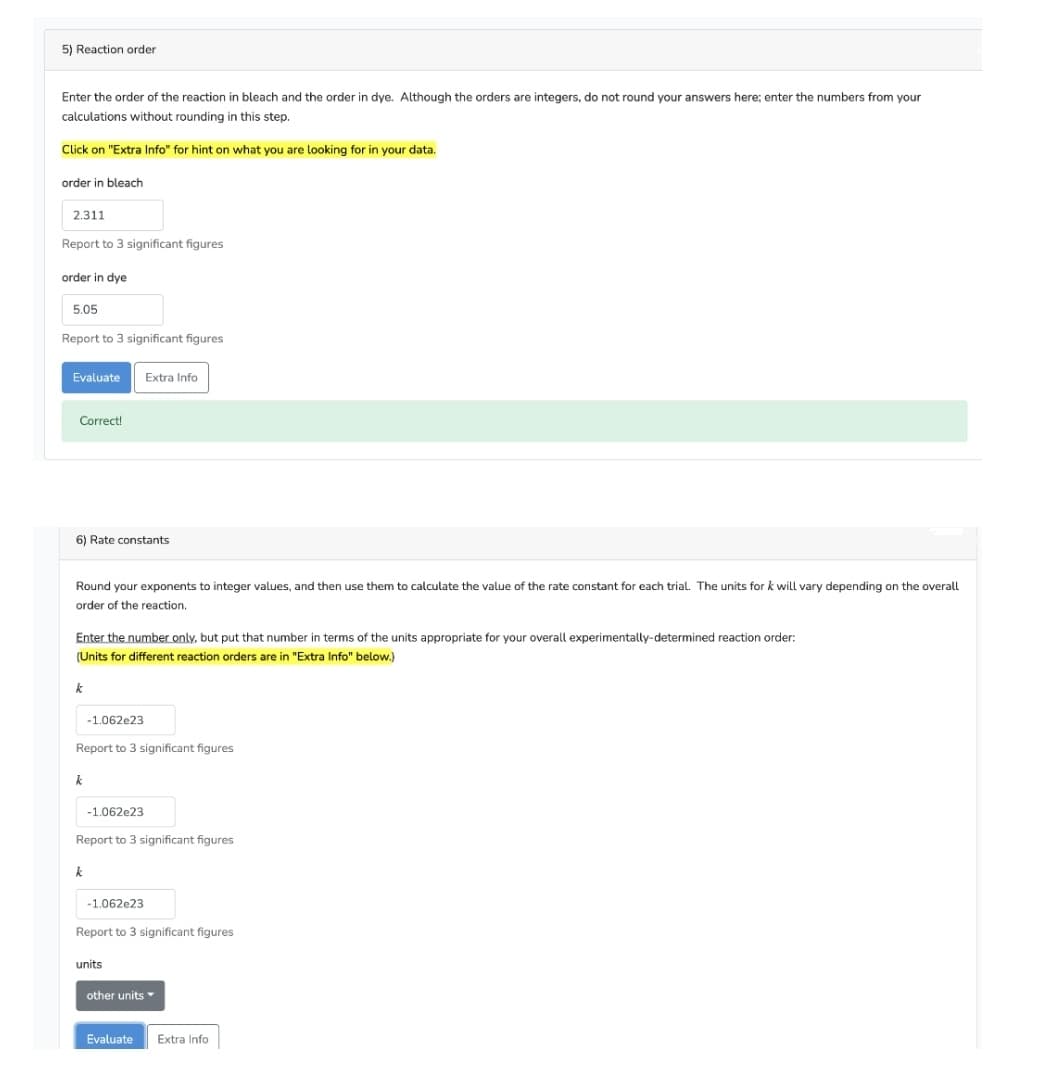
Transcribed Image Text:5) Reaction order
Enter the order of the reaction in bleach and the order in dye. Although the orders are integers, do not round your answers here; enter the numbers from your
calculations without rounding in this step.
Click on "Extra Info" for hint on what you are looking for in your data.
order in bleach
2.311
Report to 3 significant figures
order in dye
5.05
Report to 3 significant figures
Evaluate
Extra Info
Correct!
6) Rate constants
Round your exponents to integer values, and then use them to calculate the value of the rate constant for each trial. The units for k will vary depending on the overall
order of the reaction.
Enter the number only, but put that number in terms of the units appropriate for your overall experimentally-determined reaction order:
(Units for different reaction orders are in "Extra Info" below.)
k
-1.062e23
Report to 3 significant figures
k
-1.062e23
Report to 3 significant figures
k
-1.062e23
Report to 3 significant figures
units
other units
Evaluate Extra Info
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning