5. A water supply company, One Team, found out that their water supply had been contaminated with a sparingly soluble magnesium salt. They requested the help of Magent Analytical Laboratory to identify the magnesium salt. They chose to identify this using Ksp determination after narrowing it down to 2 salts: Salt MgCO3 Mg(OH)2 Theoretical Ksp 3.5 x 10-8 1.8 x 10-11 For the standardization of the HCl titrant, 5.230 mg Na2CO3 (98.9% purity, MW = 105.99 g/mol) required 24.4 mL of the titrant to reach the phenolphthalein endpoint. After isolating and precipitating the magnesium salt A, a portion was dissolved in distilled water in order to make a 250.0-mL saturated solution. The titration of a 50.0-mL aliquot of the saturated solution required 8.20 mL of HCl titrant to reach the phenolphthalein endpoint. Meanwhile, dissolving this magnesium salt in 250.0 mL of 0.0200 M MgCl2 required 1.51 mL of the same HCl titrant to titrate a 100.0-mL solution. a. What is the molarity of the HCl titrant? b. Comparing the data in distilled water with the two possible solids, which is the most likely identity of the magnesium salt? Show your full solutions in getting your conclusion. Note: Don't forget to factor in stoichiometry c. What is the average experimental Ksp for both solvents (distilled water and 0.0200 M MgCl2) and individual solubility in each solvent of the magnesium salt?
5. A water supply company, One Team, found out that their water supply had been contaminated with a sparingly soluble magnesium salt. They requested the help of Magent Analytical Laboratory to identify the magnesium salt. They chose to identify this using Ksp determination after narrowing it down to 2 salts: Salt MgCO3 Mg(OH)2 Theoretical Ksp 3.5 x 10-8 1.8 x 10-11 For the standardization of the HCl titrant, 5.230 mg Na2CO3 (98.9% purity, MW = 105.99 g/mol) required 24.4 mL of the titrant to reach the phenolphthalein endpoint. After isolating and precipitating the magnesium salt A, a portion was dissolved in distilled water in order to make a 250.0-mL saturated solution. The titration of a 50.0-mL aliquot of the saturated solution required 8.20 mL of HCl titrant to reach the phenolphthalein endpoint. Meanwhile, dissolving this magnesium salt in 250.0 mL of 0.0200 M MgCl2 required 1.51 mL of the same HCl titrant to titrate a 100.0-mL solution. a. What is the molarity of the HCl titrant? b. Comparing the data in distilled water with the two possible solids, which is the most likely identity of the magnesium salt? Show your full solutions in getting your conclusion. Note: Don't forget to factor in stoichiometry c. What is the average experimental Ksp for both solvents (distilled water and 0.0200 M MgCl2) and individual solubility in each solvent of the magnesium salt?
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.25QAP
Related questions
Question
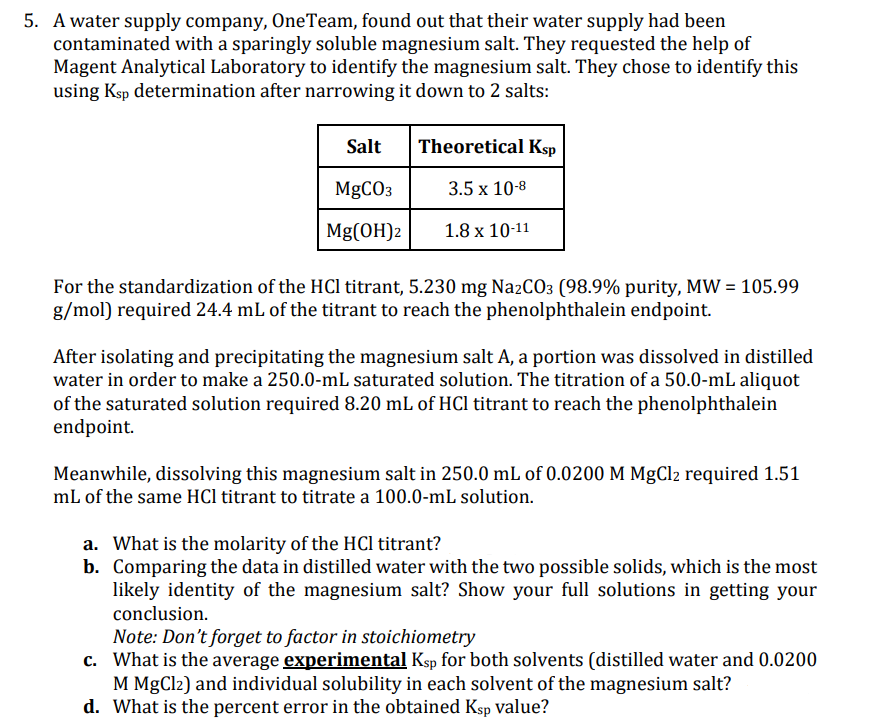
Transcribed Image Text:5. A water supply company, One Team, found out that their water supply had been
contaminated with a sparingly soluble magnesium salt. They requested the help of
Magent Analytical Laboratory to identify the magnesium salt. They chose to identify this
using Ksp determination after narrowing it down to 2 salts:
Salt
MgCO3
Mg(OH)2
Theoretical Ksp
3.5 x 10-8
1.8 x 10-11
For the standardization of the HCl titrant, 5.230 mg Na2CO3 (98.9% purity, MW = 105.99
g/mol) required 24.4 mL of the titrant to reach the phenolphthalein endpoint.
After isolating and precipitating the magnesium salt A, a portion was dissolved in distilled
water in order to make a 250.0-mL saturated solution. The titration of a 50.0-mL aliquot
of the saturated solution required 8.20 mL of HCl titrant to reach the phenolphthalein
endpoint.
Meanwhile, dissolving this magnesium salt in 250.0 mL of 0.0200 M MgCl2 required 1.51
mL of the same HCl titrant to titrate a 100.0-mL solution.
a. What is the molarity of the HCl titrant?
b. Comparing the data in distilled water with the two possible solids, which is the most
likely identity of the magnesium salt? Show your full solutions in getting your
conclusion.
Note: Don't forget to factor in stoichiometry
c.
What is the average experimental Ksp for both solvents (distilled water and 0.0200
M MgCl2) and individual solubility in each solvent of the magnesium salt?
d. What is the percent error in the obtained Ksp value?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning