5. Derive the nth-order reaction kinetics of a B species, starting from CBo to CB with initial time at t = 0 and final time, t. (Hint: Use the general form from number 3).
5. Derive the nth-order reaction kinetics of a B species, starting from CBo to CB with initial time at t = 0 and final time, t. (Hint: Use the general form from number 3).
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 50E: For the past 10 years, the unsaturated hydrocarbon 1, 3-butadiene (CH2 = CH - CH = CH2) has ranked...
Related questions
Question
100%
Please help me with number 5.
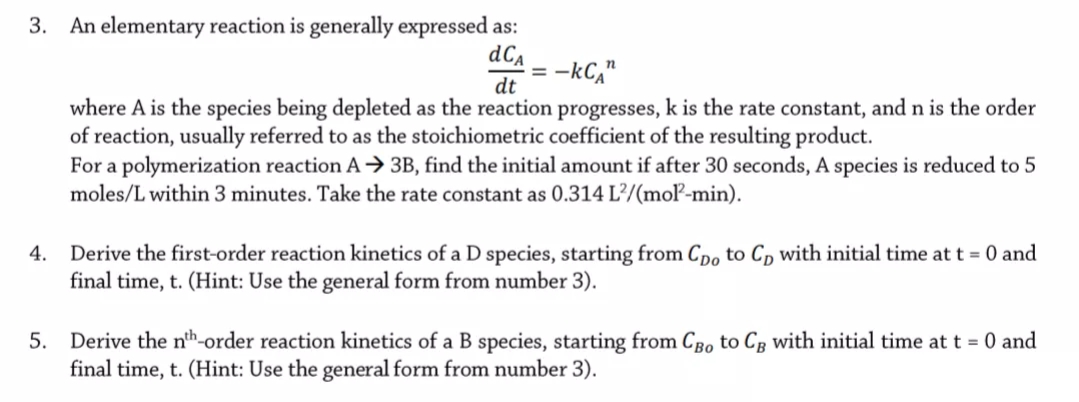
Transcribed Image Text:3. An elementary reaction is generally expressed as:
dCa
-kC,"
dt
where A is the species being depleted as the reaction progresses, k is the rate constant, and n is the order
of reaction, usually referred to as the stoichiometric coefficient of the resulting product.
For a polymerization reaction A → 3B, find the initial amount if after 30 seconds, A species is reduced to 5
moles/L within 3 minutes. Take the rate constant as 0.314 L²/(moľ-min).
Derive the first-order reaction kinetics of a D species, starting from Cpo to Cp with initial time at t = 0 and
final time, t. (Hint: Use the general form from number 3).
4.
Derive the nth-order reaction kinetics of a B species, starting from CBo to Cg with initial time at t = 0 and
final time, t. (Hint: Use the general form from number 3).
5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
