The smog constituent peroxyacetyl nitrate (PAN) dissociates into peroxyacetyl radicals and NO2(g) in a first order reaction with a half- life of 32 min. CH;COONO, (3) CH3COO • (g) + NO2(g) PAN Peroxyacetyl radical (a) What is the value of the rate constant? (b) If the initial concentration of PAN in an air sample is 2.7x1015 molecules/L, what will be the concentration 2.24 h later?
The smog constituent peroxyacetyl nitrate (PAN) dissociates into peroxyacetyl radicals and NO2(g) in a first order reaction with a half- life of 32 min. CH;COONO, (3) CH3COO • (g) + NO2(g) PAN Peroxyacetyl radical (a) What is the value of the rate constant? (b) If the initial concentration of PAN in an air sample is 2.7x1015 molecules/L, what will be the concentration 2.24 h later?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section14.4: Concentration-time Relationships: Integrated Rate Laws
Problem 14.5CYU: Sucrose, a sugar, decomposes in acid solution to give glucose and fructose. The reaction is...
Related questions
Question
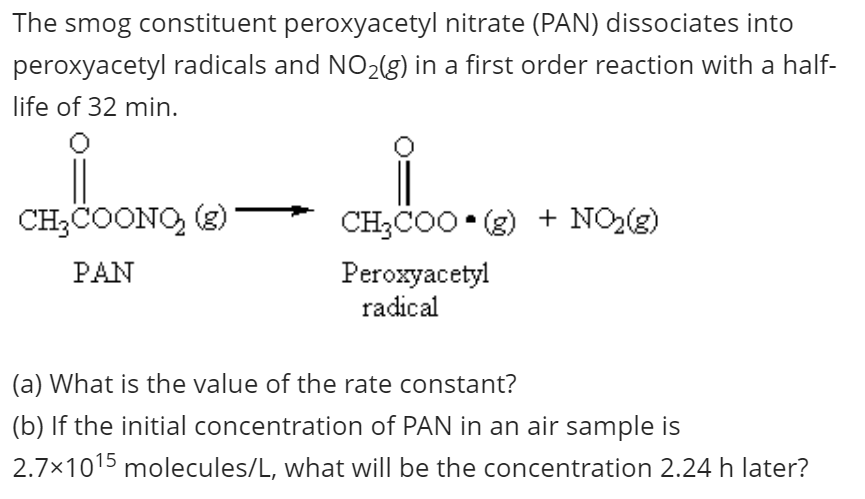
Transcribed Image Text:The smog constituent peroxyacetyl nitrate (PAN) dissociates into
peroxyacetyl radicals and NO2(g) in a first order reaction with a half-
life of 32 min.
CH3COONO, (3)
CH3CO0 • (g) + NO(g)
PAN
Peroxyacetyl
radical
(a) What is the value of the rate constant?
(b) If the initial concentration of PAN in an air sample is
2.7x1015 molecules/L, what will be the concentration 2.24 h later?
Expert Solution
Step 1
Given:
The smog constituent PAN dissociates into peroxyacetyl radicals and NO2 (g) in a first-order reaction.
We have to calculate the rate constant and the concentration of PAN after 2.24 h if the initial concentration of PAN is .
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning