5. II II Identify the type of reaction based on the given chemical equations and balance the equation afterwards. 1. NaBr + Ca(OH)2 → СаBrz + NaOH 2. NH3+ H2(SO4) → (NH4)2(SO4) 3. C5H9O + O2 CO2 + H2O -> Pb + H3(PO4) → H2 + Pb3(PO4)2 %3D 4. NH4 (NO3) → LINO3 + (NH4)3N = %3D Li3N + - HBr + Al(OH)3 → H20 + AIBR3
5. II II Identify the type of reaction based on the given chemical equations and balance the equation afterwards. 1. NaBr + Ca(OH)2 → СаBrz + NaOH 2. NH3+ H2(SO4) → (NH4)2(SO4) 3. C5H9O + O2 CO2 + H2O -> Pb + H3(PO4) → H2 + Pb3(PO4)2 %3D 4. NH4 (NO3) → LINO3 + (NH4)3N = %3D Li3N + - HBr + Al(OH)3 → H20 + AIBR3
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 15CR: List and define all the ways of classifying chemical reactions that have been discussed in the text....
Related questions
Question
100%
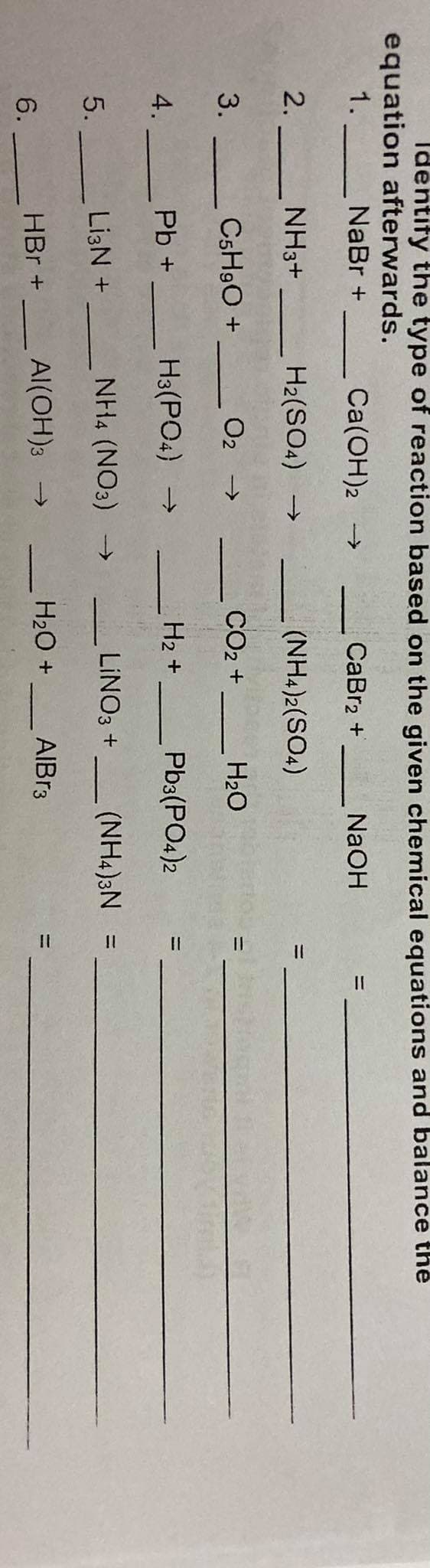
Transcribed Image Text:Identify the type of reaction based on the given chemical equations and balance the
equation afterwards.
NaBr +
1.
Ca(OH)2 →
CaBr2 +
NaOH
%3D
2. -
NH3+
H2(SO4) →
(NH4)2(SO4)
3.
C5H9O +
O2 >
CO2 +
H20
H3(PO4) →
H2 +
Pb3(PO4)2
%3D
4.
Pb +
NH4 (NO3) -→
LINO3 +
(NH4)3N =
%3D
5.
Li3N +
HBr +
Al(OH)3 →
H20 +
AIBR3
6.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning