5. Molar concentration of iron salicylate ion in 50 mL volumetric flask (calculated using equation of line) 6. Molar concentration of iron salicylate ion in 250 mL volumetric flask (parent aspirin stock solution) 7. Moles of iron salicylate ion in 250 mL volumetric flask (parent aspirin stock solution)
5. Molar concentration of iron salicylate ion in 50 mL volumetric flask (calculated using equation of line) 6. Molar concentration of iron salicylate ion in 250 mL volumetric flask (parent aspirin stock solution) 7. Moles of iron salicylate ion in 250 mL volumetric flask (parent aspirin stock solution)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
help needed for answering the following last 5 questions, any help is appreciated! :)
![The molar mass of acetylsalicylic acid is 180.16 g mol 1
Part A: Mass of acetylsalicylic acid data and molar concentration calculation
Unrounded
Rounded
Unit
Mass of acetylsalicylic acid in 1 L
1.5739
g
solution
1. Molar concentration of acetylsalicylic
0.00876310
0.00876
mol L-1
acid
Part A: Volume data, ferric salicylate ion concentration calculation and absorbance data
Trial
Volume of solution
Final volume
2. [Fe-salicylate ion] (mol L¯!)
Absorbance
S (mL)
(mL)
Unrounded
Rounded
Blank (
FeCl3
soln used
0.00
50.00
0.00000
0.00
0.000
to calibrate
colorimeter)
1
2.00
50.00
3.50500 x 104
3.51 x 104
0.261
3.00
50.00
5.25700 x 104
5.26 x 10-4
0.376
3
4.00
50.00
7.01000 x 104
7.01 x 104
0.523
5.00
50.00
8.76300 x 104
8.76 x 104
0.656
6.00
50.00
0.00105150
1.05 x 103
0.778
Part A: Slope and intercept calculation
Unrounded
Rounded
Unit
3. Slope
749.750
7.50 × 102
L mol-
-0.00241872
4. y-intercept
6.80000× 103
-0.00242
Unitless](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff8ca914a-44f4-4fe1-bd26-de14ecc5b978%2F1c8f78c4-27c2-4ab8-aa3a-edfcb1966e03%2F12l5yet_processed.jpeg&w=3840&q=75)
Transcribed Image Text:The molar mass of acetylsalicylic acid is 180.16 g mol 1
Part A: Mass of acetylsalicylic acid data and molar concentration calculation
Unrounded
Rounded
Unit
Mass of acetylsalicylic acid in 1 L
1.5739
g
solution
1. Molar concentration of acetylsalicylic
0.00876310
0.00876
mol L-1
acid
Part A: Volume data, ferric salicylate ion concentration calculation and absorbance data
Trial
Volume of solution
Final volume
2. [Fe-salicylate ion] (mol L¯!)
Absorbance
S (mL)
(mL)
Unrounded
Rounded
Blank (
FeCl3
soln used
0.00
50.00
0.00000
0.00
0.000
to calibrate
colorimeter)
1
2.00
50.00
3.50500 x 104
3.51 x 104
0.261
3.00
50.00
5.25700 x 104
5.26 x 10-4
0.376
3
4.00
50.00
7.01000 x 104
7.01 x 104
0.523
5.00
50.00
8.76300 x 104
8.76 x 104
0.656
6.00
50.00
0.00105150
1.05 x 103
0.778
Part A: Slope and intercept calculation
Unrounded
Rounded
Unit
3. Slope
749.750
7.50 × 102
L mol-
-0.00241872
4. y-intercept
6.80000× 103
-0.00242
Unitless
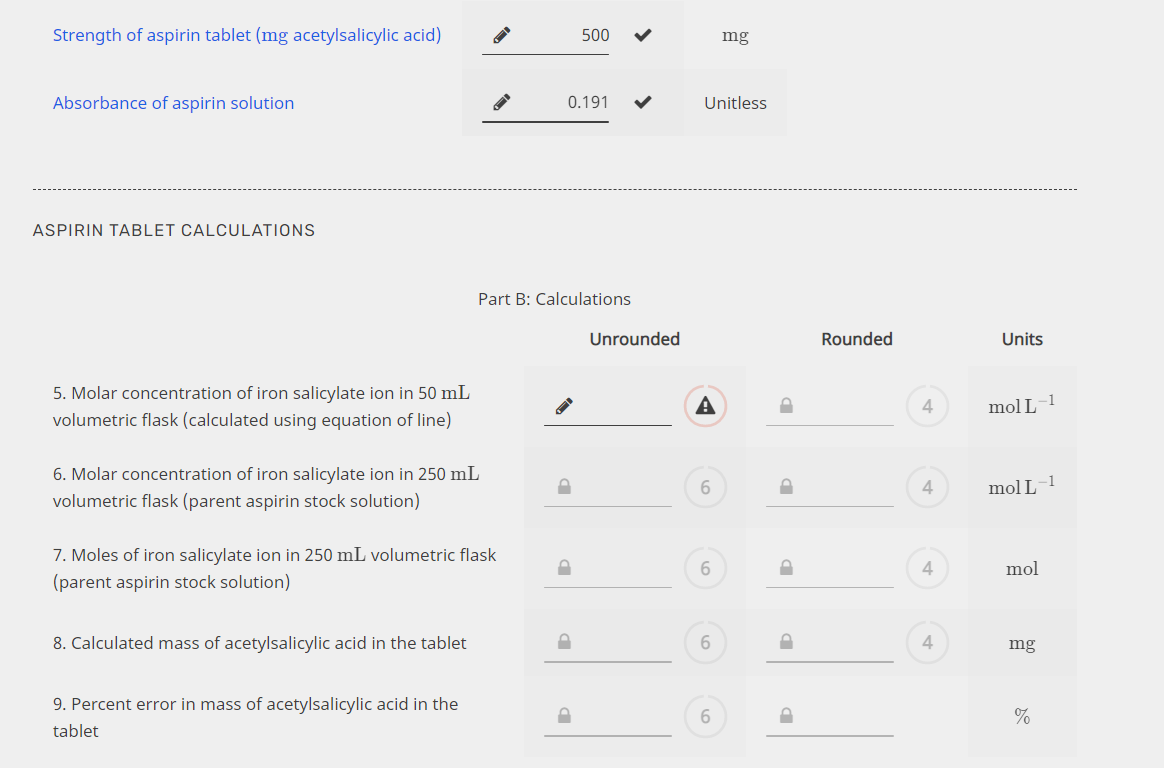
Transcribed Image Text:Strength of aspirin tablet (mg acetylsalicylic acid)
500
mg
Absorbance of aspirin solution
0.191
Unitless
ASPIRIN TABLET CALCULATIONS
Part B: Calculations
Unrounded
Rounded
Units
5. Molar concentration of iron salicylate ion in 50 mL
volumetric flask (calculated using equation of line)
A
4
mol L
6. Molar concentration of iron salicylate ion in 250 mL
volumetric flask (parent aspirin stock solution)
6.
4
mol L-1
7. Moles of iron salicylate ion in 250 mL volumetric flask
mol
(parent aspirin stock solution)
8. Calculated mass of acetylsalicylic acid in the tablet
6.
mg
9. Percent error in mass of acetylsalicylic acid in the
%
tablet
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

