5. Ribose's structure is OH HO ОН ОН OH OH a) Draw using the arrow formalism the reaction of linear ribose to make a 5-membered ring. b) What is the name of the resulting functional group? c) Given that reaction of alcohols (such as RCH2OH) with carbonyls (such as HCO-R') to create this functional group is typically not thermodynamically favored, why does most ribose in water exist as a 5-membered ring? A few word answer is fine. d) AGfor thir roction ic about 5 k/mol Assuming that vou start with mM colution of
5. Ribose's structure is OH HO ОН ОН OH OH a) Draw using the arrow formalism the reaction of linear ribose to make a 5-membered ring. b) What is the name of the resulting functional group? c) Given that reaction of alcohols (such as RCH2OH) with carbonyls (such as HCO-R') to create this functional group is typically not thermodynamically favored, why does most ribose in water exist as a 5-membered ring? A few word answer is fine. d) AGfor thir roction ic about 5 k/mol Assuming that vou start with mM colution of
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter21: Biochemistry
Section: Chapter Questions
Problem 36QAP
Related questions
Question
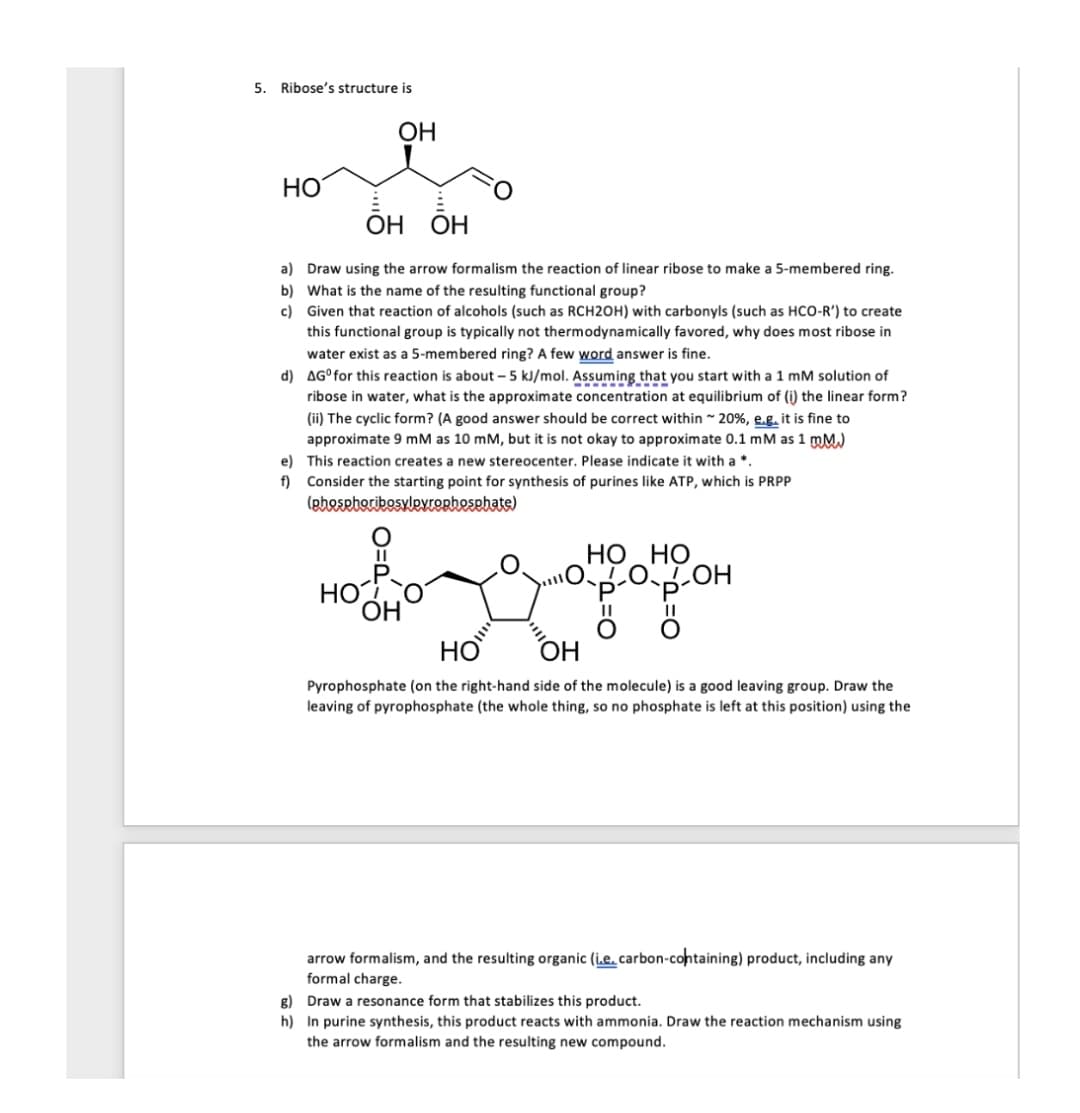
Transcribed Image Text:5. Ribose's structure is
ОН
HO
ОН ОН
a) Draw using the arrow formalism the reaction of linear ribose to make a 5-membered ring.
b) What is the name of the resulting functional group?
c) Given that reaction of alcohols (such as RCH2OH) with carbonyls (such as HCO-R') to create
this functional group is typically not thermodynamically favored, why does most ribose in
water exist as a 5-membered ring? A few word answer is fine.
d) AG°for this reaction is about - 5 kJ/mol. Assuming that you start with a 1 mM solution of
ribose in water, what is the approximate concentration at equilibrium of (i) the linear form?
(ii) The cyclic form? (A good answer should be correct within 20%, e.g. it is fine to
approximate 9 mM as 10 mM, but it is not okay to approximate 0.1 mM as 1 mM.)
e) This reaction creates a new stereocenter. Please indicate it with a *.
f) Consider the starting point for synthesis of purines like ATP, which is PRPP
(pheseboribosylevOrhesehate)
НО НО
LOLOH
HO
ОН
HO
OH
Pyrophosphate (on the right-hand side of the molecule) is a good leaving group. Draw the
leaving of pyrophosphate (the whole thing, so no phosphate is left at this position) using the
arrow formalism, and the resulting organic (i.e. carbon-containing) product, including any
formal charge.
g) Draw a resonance form that stabilizes this product.
h) In purine synthesis, this product reacts with ammonia. Draw the reaction mechanism using
the arrow formalism and the resulting new compound.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning