5. The reaction of glucose synthesis is endergonic (6CO2 + 6H2O + energy C6H1206 + 602), AG = + 686 kcal/mole, which mean that it is not spontaneous. How is it possible that this reaction still takes place in living organisms? * The endergonic glucose synthesis is coupled with an exergonic reaction (for example ATP hydrolysis) in the same system. The endergonic glucose synthesis is coupled with other endergonic reactions in the same system. The exergonic glucose synthesi io onunl
5. The reaction of glucose synthesis is endergonic (6CO2 + 6H2O + energy C6H1206 + 602), AG = + 686 kcal/mole, which mean that it is not spontaneous. How is it possible that this reaction still takes place in living organisms? * The endergonic glucose synthesis is coupled with an exergonic reaction (for example ATP hydrolysis) in the same system. The endergonic glucose synthesis is coupled with other endergonic reactions in the same system. The exergonic glucose synthesi io onunl
Biology 2e
2nd Edition
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:Matthew Douglas, Jung Choi, Mary Ann Clark
Chapter6: Metabolism
Section: Chapter Questions
Problem 7RQ: Which of the following comparisons or contrasts between endergonic and exergonic reactions is false?...
Related questions
Question
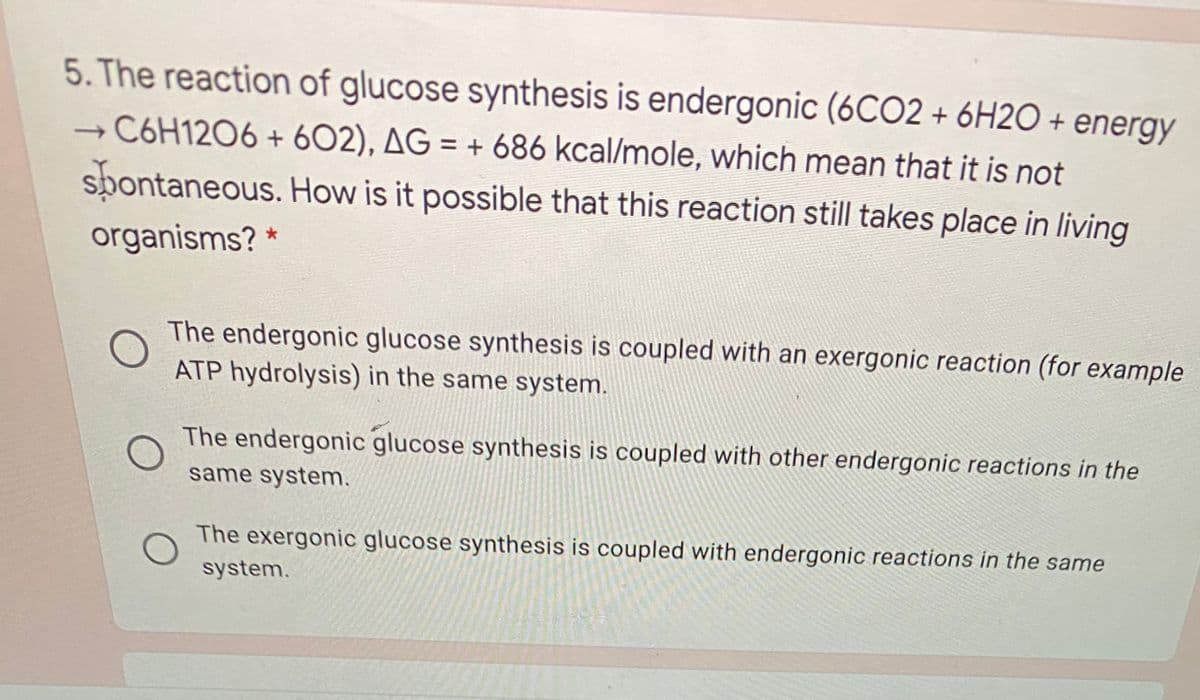
Transcribed Image Text:5. The reaction of glucose synthesis is endergonic (6CO2 + 6H2O + energy
C6H12O6 + 602), AG = + 686 kcal/mole, which mean that it is not
spontaneous. How is it possible that this reaction still takes place in living
organisms? *
The endergonic glucose synthesis is coupled with an exergonic reaction (for example
ATP hydrolysis) in the same system.
The endergonic glucose synthesis is coupled with other endergonic reactions in the
same system.
The exergonic glucose synthesis is coupled with endergonic reactions in the same
system.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning