5. This quarter included discussion of an experiment that involved the formation of two semicarbazones by the reaction of cyclohexanone (A) and 2-furaldehyde (B) with semicarbazide (C). By varying the reagents and conditions used, the effects of kinetic versus thermodynamic control in the formation of these semicarbizones could be demonstrated. a. Write the mechanism for the formation of cyclohexanone semicarbazone. b. In the first portion of the experiment, you reacted A and B in separate containers with C then measured the melting points of the products formed. Explain why you performed this portion of the experiment. H. NH2 H. А B c. You reacted A and B – in the same container – with C at three different temperatures – below, at, and above room temperature. Explain how the results of these reactions allowed you to determine which semicarbizone was formed under kinetic control and which was formed under thermodynamic control. d. In the final portion of the experiment, you performed two reactions, the results of which were used to confirm whether the kinetic and thermodynamic products were correctly identified in the previous portion of the experiment. Explain how the results the reactions were used to confirm the identities of the products.
5. This quarter included discussion of an experiment that involved the formation of two semicarbazones by the reaction of cyclohexanone (A) and 2-furaldehyde (B) with semicarbazide (C). By varying the reagents and conditions used, the effects of kinetic versus thermodynamic control in the formation of these semicarbizones could be demonstrated. a. Write the mechanism for the formation of cyclohexanone semicarbazone. b. In the first portion of the experiment, you reacted A and B in separate containers with C then measured the melting points of the products formed. Explain why you performed this portion of the experiment. H. NH2 H. А B c. You reacted A and B – in the same container – with C at three different temperatures – below, at, and above room temperature. Explain how the results of these reactions allowed you to determine which semicarbizone was formed under kinetic control and which was formed under thermodynamic control. d. In the final portion of the experiment, you performed two reactions, the results of which were used to confirm whether the kinetic and thermodynamic products were correctly identified in the previous portion of the experiment. Explain how the results the reactions were used to confirm the identities of the products.
Chapter19: Aldehydes And Ketones: Nucleophilic Addition Reactions
Section19.SE: Something Extra
Problem 38MP
Related questions
Question
please help me - please
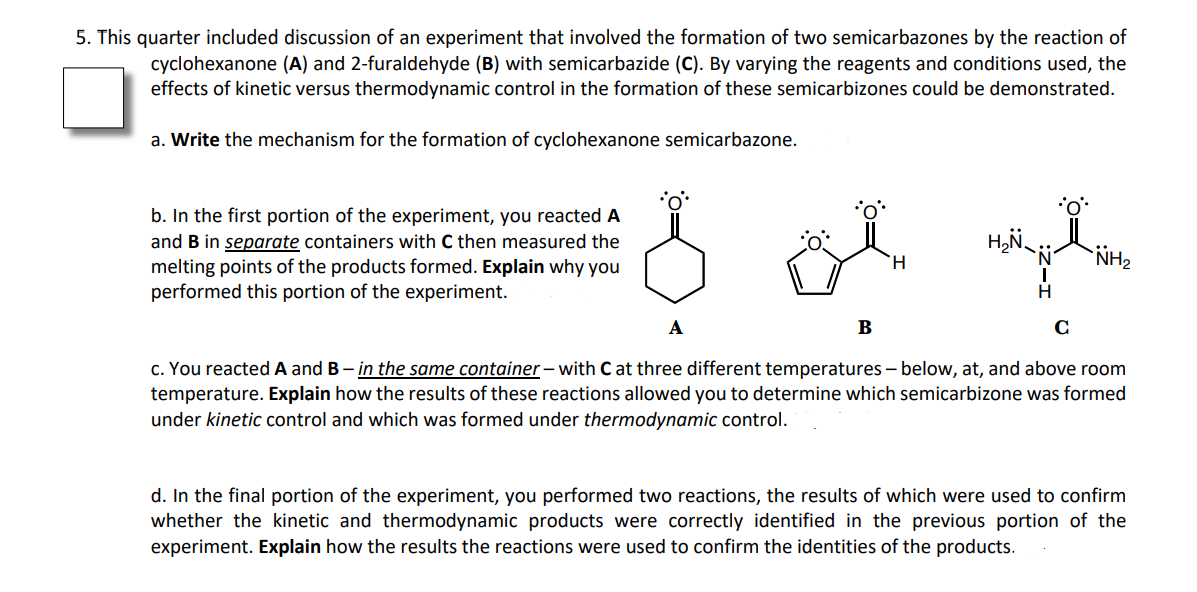
Transcribed Image Text:5. This quarter included discussion of an experiment that involved the formation of two semicarbazones by the reaction of
cyclohexanone (A) and 2-furaldehyde (B) with semicarbazide (C). By varying the reagents and conditions used, the
effects of kinetic versus thermodynamic control in the formation of these semicarbizones could be demonstrated.
a. Write the mechanism for the formation of cyclohexanone semicarbazone.
b. In the first portion of the experiment, you reacted A
and B in separate containers with C then measured the
NH2
H.
melting points of the products formed. Explain why you
performed this portion of the experiment.
H
A
B
C
c. You reacted A and B– in the same container– with C at three different temperatures– below, at, and above room
temperature. Explain how the results of these reactions allowed you to determine which semicarbizone was formed
under kinetic control and which was formed under thermodynamic control.
d. In the final portion of the experiment, you performed two reactions, the results of which were used to confirm
whether the kinetic and thermodynamic products were correctly identified in the previous portion of the
experiment. Explain how the results the reactions were used to confirm the identities of the products.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

