50 > A 0.775 g sample of a metal, M, reacts completely with sulfuric acid according to M(s) + H,SO,(aq) → MSO,(aq) + H,(g) A volume of 301 mL of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. Atmospheric pressure is 756.0 Torr, and the temperature is 25 °C. Calculate the molar mass of the metal. g/mol 63.29 molar mass: Question Source: MRG-General Chemistry | Publisher: Univer help contact us terms of use wbout os privacy policy careers MacBook Pro
50 > A 0.775 g sample of a metal, M, reacts completely with sulfuric acid according to M(s) + H,SO,(aq) → MSO,(aq) + H,(g) A volume of 301 mL of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. Atmospheric pressure is 756.0 Torr, and the temperature is 25 °C. Calculate the molar mass of the metal. g/mol 63.29 molar mass: Question Source: MRG-General Chemistry | Publisher: Univer help contact us terms of use wbout os privacy policy careers MacBook Pro
Chapter5: Gases
Section: Chapter Questions
Problem 152CP: You have an equimolar mixture of the gases SO2 and O2, along with some He, in a container fitted...
Related questions
Question
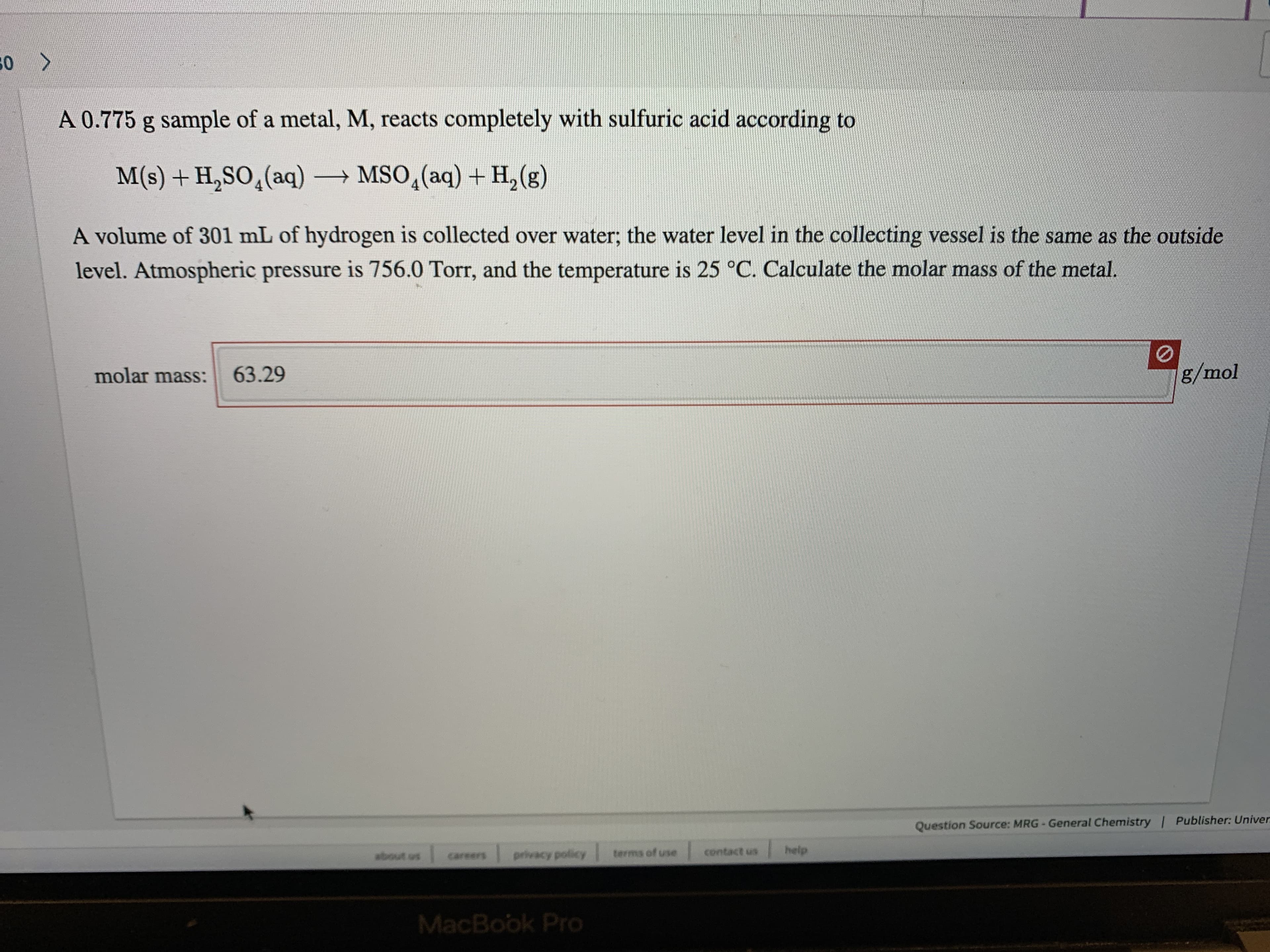
Transcribed Image Text:50 >
A 0.775 g sample of a metal, M, reacts completely with sulfuric acid according to
M(s) + H,SO,(aq) → MSO,(aq) + H,(g)
A volume of 301 mL of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside
level. Atmospheric pressure is 756.0 Torr, and the temperature is 25 °C. Calculate the molar mass of the metal.
g/mol
63.29
molar mass:
Question Source: MRG-General Chemistry | Publisher: Univer
help
contact us
terms of use
wbout os
privacy policy
careers
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning