The reaction of metallic aluminum with aqueous hydrochloric acid produces hydrogen gas. 2 Al(s) + 6 HCI(aq) → 2 AICI,(aq) + 3 H,(g) Hydrogen gas produced by this reaction is typically collected via water displacement, during which time the hydrogen gas becomes saturated with water vapor. A 220.0 mL sample of gas with a total pressure 149 kPa was collected via water displacement at 29.4 °C. Calculate the partial pressure of hydrogen gas in the sample. The vapor pressure of water at 29.4 °C is 4.10 kPa. PH, kPa Calculate the mass of aluminum that reacted to produce this quantity of hydrogen gas. mass:
The reaction of metallic aluminum with aqueous hydrochloric acid produces hydrogen gas. 2 Al(s) + 6 HCI(aq) → 2 AICI,(aq) + 3 H,(g) Hydrogen gas produced by this reaction is typically collected via water displacement, during which time the hydrogen gas becomes saturated with water vapor. A 220.0 mL sample of gas with a total pressure 149 kPa was collected via water displacement at 29.4 °C. Calculate the partial pressure of hydrogen gas in the sample. The vapor pressure of water at 29.4 °C is 4.10 kPa. PH, kPa Calculate the mass of aluminum that reacted to produce this quantity of hydrogen gas. mass:
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.93PAE: 93 The complete combustion of octane can be used as a model for the burning of gasoline:...
Related questions
Question
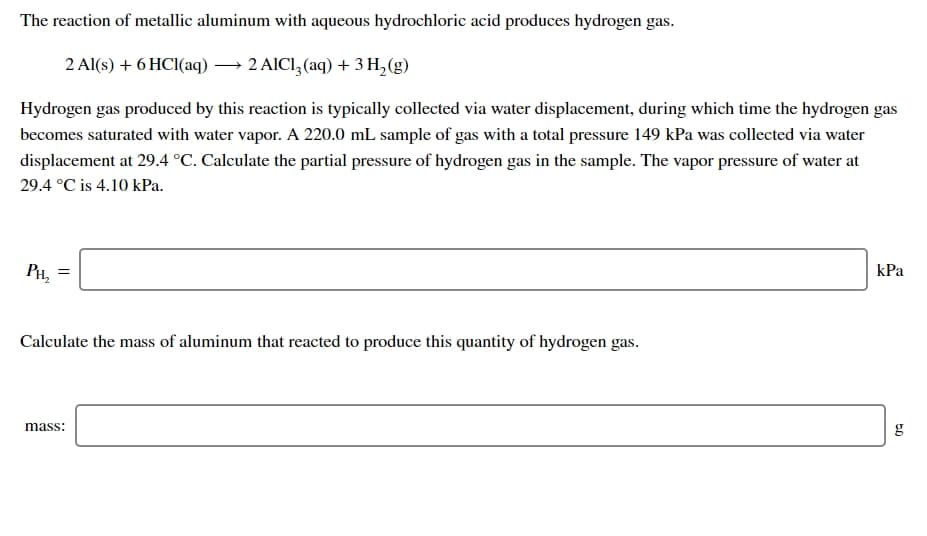
Transcribed Image Text:The reaction of metallic aluminum with aqueous hydrochloric acid produces hydrogen gas.
2 Al(s) + 6 HCI(aq) → 2 AICI, (aq) + 3 H,(g)
Hydrogen gas produced by this reaction is typically collected via water displacement, during which time the hydrogen gas
becomes saturated with water vapor. A 220.0 mL sample of gas with a total pressure 149 kPa was collected via water
displacement at 29.4 °C. Calculate the partial pressure of hydrogen gas in the sample. The vapor pressure of water at
29.4 °C is 4.10 kPa.
PH, =
kPa
Calculate the mass of aluminum that reacted to produce this quantity of hydrogen gas.
mass:
g
Expert Solution
Step 1
The balanced reaction taking place is given as,
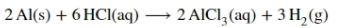
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning