5:07 PM Wed Feb 12 n 66% < 88 Q chem 1b readings chem 1b readings chem 1B discussion Chem 1b Lectures (27) Choose the substance with the lowest surface tension. (A) CH3OH (C) C6H6 Q) H20 (B) CH3CH2CH2CH3 TE) (CH3)2CO is surfece (28) Identify the compound that does not have dipole-dipole forces as its strongest force. tension caen us/B? why A) CH2B12 B) CH30CH3 CH3CI (В НСВr3 (E))CO2 34.6°C 78.3°C 100°C 800 760 Normal boiling point Diethyl ther 600
5:07 PM Wed Feb 12 n 66% < 88 Q chem 1b readings chem 1b readings chem 1B discussion Chem 1b Lectures (27) Choose the substance with the lowest surface tension. (A) CH3OH (C) C6H6 Q) H20 (B) CH3CH2CH2CH3 TE) (CH3)2CO is surfece (28) Identify the compound that does not have dipole-dipole forces as its strongest force. tension caen us/B? why A) CH2B12 B) CH30CH3 CH3CI (В НСВr3 (E))CO2 34.6°C 78.3°C 100°C 800 760 Normal boiling point Diethyl ther 600
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 3ALQ
Related questions
Question
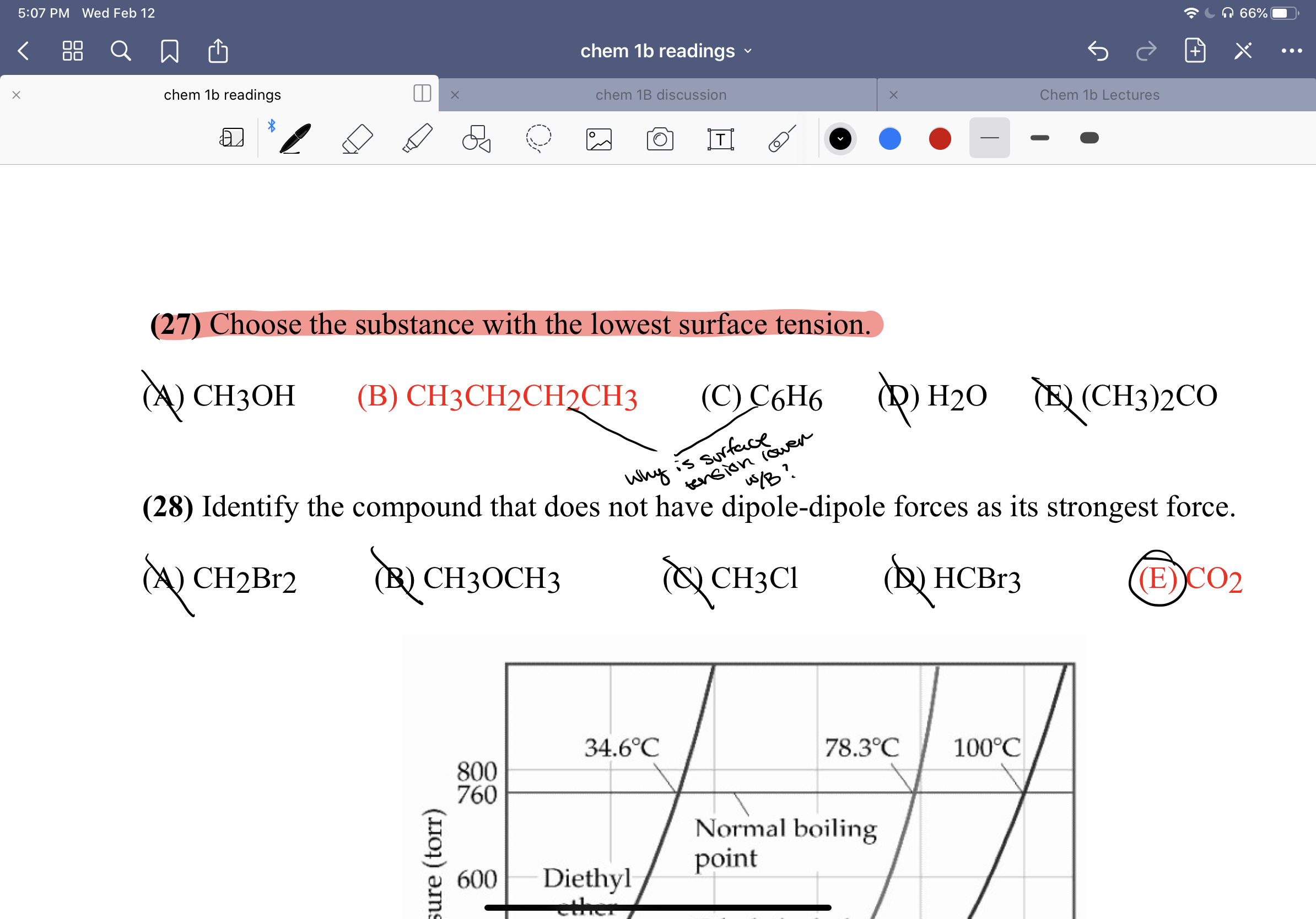
I do not understand why in this case B would have lower surface tension than C. Can someone please explain it to me. Question shown below and highlighted in red.
Transcribed Image Text:5:07 PM Wed Feb 12
n 66%
< 88 Q
chem 1b readings
chem 1b readings
chem 1B discussion
Chem 1b Lectures
(27) Choose the substance with the lowest surface tension.
(A) CH3OH
(C) C6H6
Q) H20
(B) CH3CH2CH2CH3
TE) (CH3)2CO
is surfece
(28) Identify the compound that does not have dipole-dipole forces as its strongest force.
tension caen
us/B?
why
A) CH2B12
B) CH30CH3
CH3CI
(В НСВr3
(E))CO2
34.6°C
78.3°C
100°C
800
760
Normal boiling
point
Diethyl
ther
600
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax