5880 grams of paraffin wax, C25H52 are burned in excess oxygen at 520°C in a rigid 150 Liter container according to the following equation: C25H52 + O2 (g) → CO2 (g) + H2O(g) a) Balance the equation b) Calculate the pressure of carbon dioxide gas when the reaction goes to completion c) Calculate the pressure of water vapor when the reaction goes to completion d) If there are 18.55 moles of oxygen gas left over in the container along with the carbon dioxide and water vapor when the reaction gocs to completion, what is the total pressure of the system? e) The rigid container is opened, explain which gas is going to effuse faster, water vapor or oxygen gas? Prove mathematically how much faster.
5880 grams of paraffin wax, C25H52 are burned in excess oxygen at 520°C in a rigid 150 Liter container according to the following equation: C25H52 + O2 (g) → CO2 (g) + H2O(g) a) Balance the equation b) Calculate the pressure of carbon dioxide gas when the reaction goes to completion c) Calculate the pressure of water vapor when the reaction goes to completion d) If there are 18.55 moles of oxygen gas left over in the container along with the carbon dioxide and water vapor when the reaction gocs to completion, what is the total pressure of the system? e) The rigid container is opened, explain which gas is going to effuse faster, water vapor or oxygen gas? Prove mathematically how much faster.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.60PAE: 60 Automakers are always investigating reactions for the generation of gas to inflate air bags, in...
Related questions
Question
clear work for a,b,c,d, and e please
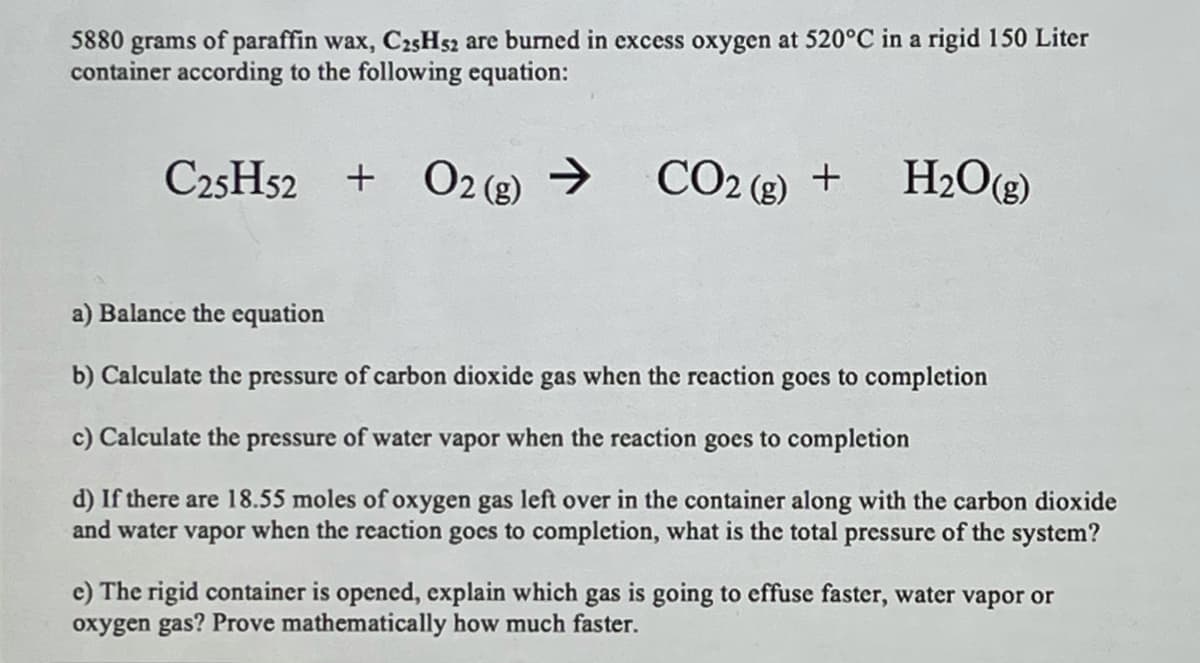
Transcribed Image Text:5880 grams of paraffin wax, C25H52 are burned in excess oxygen at 520°C in a rigid 150 Liter
container according to the following equation:
C25H52 + O2 (g) →
>
CO2 (8) +
H2O(g)
a) Balance the equation
b) Calculate the pressure of carbon dioxide gas when the reaction goes to completion
c) Calculate the pressure of water vapor when the reaction goes to completion
d) If there are 18.55 moles of oxygen gas left over in the container along with the carbon dioxide
and water vapor when the reaction gocs to completion, what is the total pressure of the system?
e) The rigid container is opened, explain which gas is going to effuse faster, water vapor or
oxygen gas? Prove mathematically how much faster.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning