6) At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 ICI(g) = I2(g) + Cl2(g). What is the equilibrium concentration of ICl if 0.75 mol of I2 and 0.75 mol of Cl2 are initially mixed in a 2.0-L flask? A) 0.23 M B) 0.28 M C) 0.45 M D) 0.56
6) At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 ICI(g) = I2(g) + Cl2(g). What is the equilibrium concentration of ICl if 0.75 mol of I2 and 0.75 mol of Cl2 are initially mixed in a 2.0-L flask? A) 0.23 M B) 0.28 M C) 0.45 M D) 0.56
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.51QE
Related questions
Question
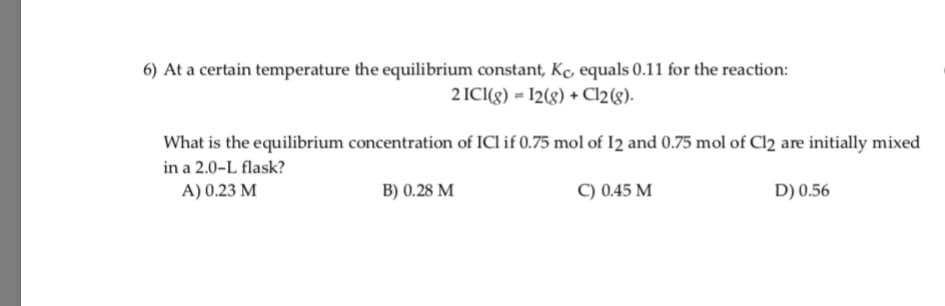
Transcribed Image Text:6) At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction:
2 ICI(g) = I2(g) + Cl2(g).
What is the equilibrium concentration of ICl if 0.75 mol of I2 and 0.75 mol of Cl2 are initially mixed
in a 2.0-L flask?
A) 0.23 M
B) 0.28 M
C) 0.45 M
D) 0.56
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning