6. For the complete redox reaction given here, (a) break down each reaction into its half reactions; (b) identify the oxidizing agent; and (c) identify the reducing agent. 2 FeCl3 + Zn → ZNC12 + 2 FeCl2
6. For the complete redox reaction given here, (a) break down each reaction into its half reactions; (b) identify the oxidizing agent; and (c) identify the reducing agent. 2 FeCl3 + Zn → ZNC12 + 2 FeCl2
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
Transcribed Image Text:B Brock Sakai : CHEM 1P91 - Fall X
b My Questions | bartleby
ChemTeam: Combustion Analys X G oxygen molar mass - Google S X
B Brock Sakai : CHEM 1P91 - Fall x +
Ims.brocku.ca/portal/site/90b45528-0b04-4bd2-85b6-897046a1e998/page/7011eaa9-4e38-4b06-9e15-1973abb58ef6
Version 264 Assign 4
1 / 2
100%
+
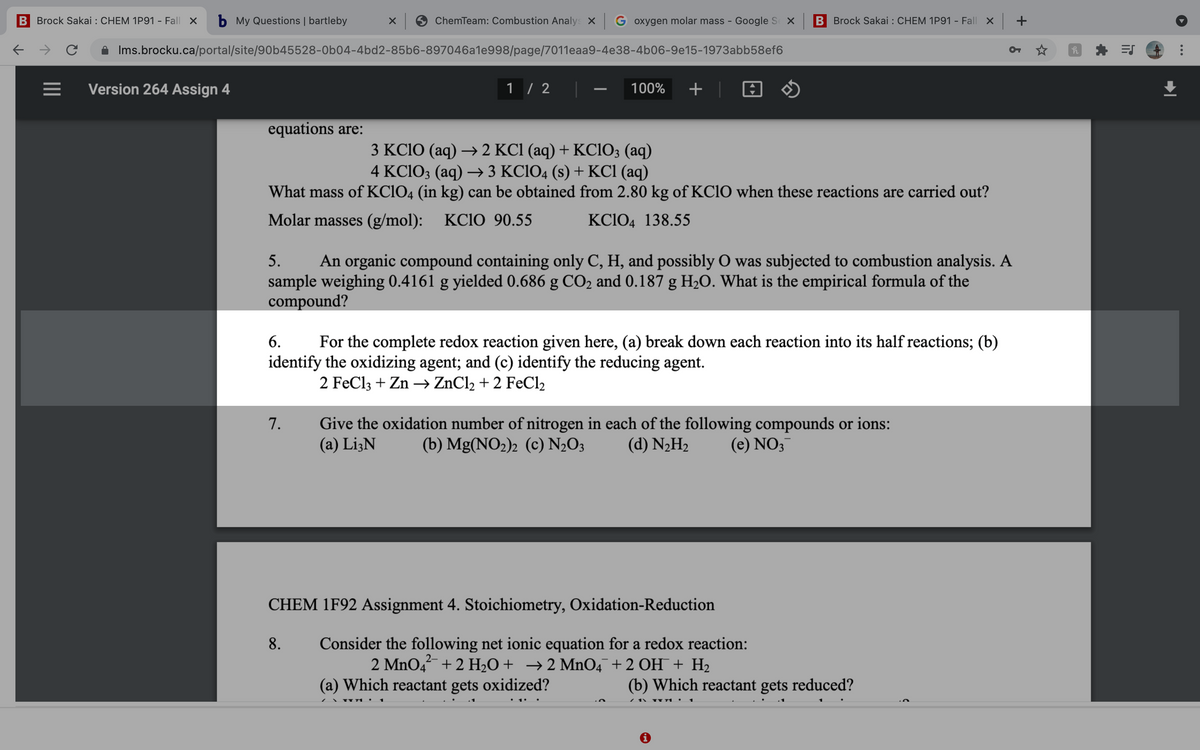
equations are:
3 KC1O (aq) → 2 KC1 (aq) + KCIO; (aq)
4 KC1O3 (aq) → 3 KC1O4 (s) + KCI (aq)
What mass of KC1O4 (in kg) can be obtained from 2.80 kg of KC1O when these reactions are carried out?
Molar masses (g/mol): KC1O 90.55
KC1O4 138.55
5.
An organic compound containing only C, H, and possibly O was subjected to combustion analysis. A
sample weighing 0.4161 g yielded 0.686 g CO2 and 0.187 g H2O. What is the empirical formula of the
compound?
6.
For the complete redox reaction given here, (a) break down each reaction into its half reactions; (b)
identify the oxidizing agent; and (c) identify the reducing agent.
2 FeCl3 + Zn –→ ZnCl2 + 2 FeCl2
Give the oxidation number of nitrogen in each of the following compounds or ions:
(a) Li3N
7.
(b) Mg(NO2)2 (c) N½O3
(d) N2H2
(e) NO3
CHEM 1F92 Assignment 4. Stoichiometry, Oxidation-Reduction
Consider the following net ionic equation for a redox reaction:
2 MnO4 + 2 H2O+ →2 MnO4¯+2 OH¯ + H2
8.
2-
(a) Which reactant gets oxidized?
(b) Which reactant gets reduced?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning