Specify which of the following are oxidation-reduction reactions, and if it is, identify the oxidizing agent, the reducing agent, the substance being oxidized, and the substance being reduced. If it is not, select No and leave the following boxes blank. Express your answers as chemical formulas. Omit states-of-matter. a. CH4 (g) + 302 (g) → CO2(g) + 2H₂O(g) Redox? Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced b. 3AgNO3(aq) + La(s) → La(NO3)3(aq) + 3Ag(s) Redox? Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced Submit Answer Try Another Version item attempt remaining
Specify which of the following are oxidation-reduction reactions, and if it is, identify the oxidizing agent, the reducing agent, the substance being oxidized, and the substance being reduced. If it is not, select No and leave the following boxes blank. Express your answers as chemical formulas. Omit states-of-matter. a. CH4 (g) + 302 (g) → CO2(g) + 2H₂O(g) Redox? Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced b. 3AgNO3(aq) + La(s) → La(NO3)3(aq) + 3Ag(s) Redox? Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced Submit Answer Try Another Version item attempt remaining
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 117GQ: Gold can be dissolved from gold-bearing rock by treating the rock with sodium cyanide in the...
Related questions
Question
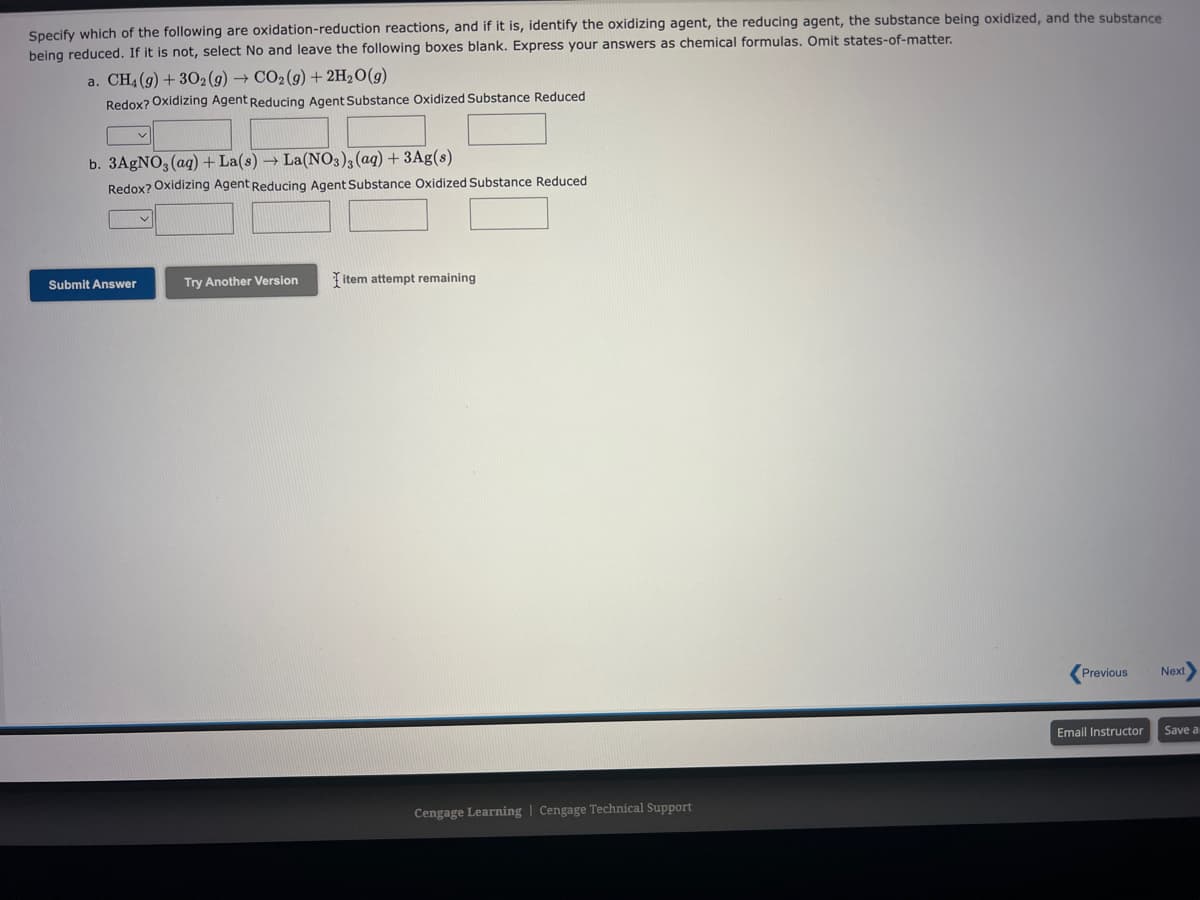
Transcribed Image Text:Specify which of the following are oxidation-reduction reactions, and if it is, identify the oxidizing agent, the reducing agent, the substance being oxidized, and the substance
being reduced. If it is not, select No and leave the following boxes blank. Express your answers as chemical formulas. Omit states-of-matter.
a. CH4 (g) + 302 (g) → CO₂(g) + 2H₂O(g)
Redox? Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced
b. 3AgNO3(aq) + La(s) → La(NO3)3(aq) + 3Ag(s)
Redox? Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced
Submit Answer
Try Another Version
item attempt remaining
Cengage Learning Cengage Technical Support
Previous
Next>
Email Instructor Save a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
