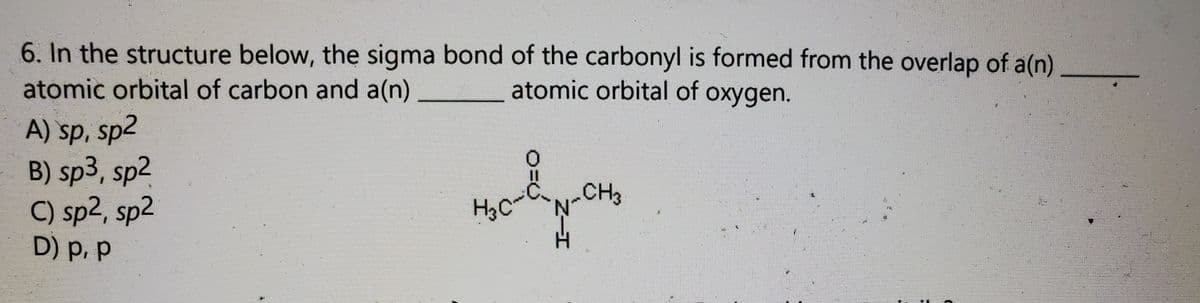
6. In the structure below, the sigma bond of the carbonyl is formed from the overlap of a(n) atomic orbital of carbon and a(n) atomic orbital of oxygen. A) sp, sp2 B) sp3, sp2 C) sp2, sp2 D) p. p CH3 H3C
Q: The pentadienyl radical, H2C“CH¬CH“CH¬CH2#, has its unpaired electron delocalized over three carbon…
A: The structure of pentadienyl radical is shown below The carbon that holds the free electron is sp2…
Q: Rizatriptan (trade name Maxalt) is a prescription drug used for the treatment of migraines. (a) How…
A: a. Rizatriptan contains two aromatic rings. b.
Q: Draw the best Lew а. NO b. O3 c. SO3 с.
A: Lewis structures, also called electron-dot structures or electron-dot diagrams, are diagrams that…
Q: 4. What is the approximate C-N-H bond angle in methanamine, according to VSEPR? O 109.5° 90° O 180 O…
A:
Q: Assign Z and E configuration to these compounds. HO HO द्र 9. The following compound A is found to…
A:
Q: Which pairs of hydrocarbons (X & Y) shown below has X with higher bo point than Y?* Y А.…
A:
Q: Below is one of the two resonance forms for the acetate ion. What is true about the bond rotations…
A: Lewis structure of a molecule can be defined as the arrangement of valence shell electrons in all…
Q: Which Newman projection accurately represents the structure shown below, looking along the a-b bond…
A:
Q: Which types of orbitals overlap to form the sigma bond between the carbon and hydrogen atoms in…
A: The compound given is,
Q: The following compound is a bioactive natural product derived from marine sponges: a) Predict the…
A:
Q: 9. The following compound A is less stable than compound B angle strain and hybridization states of…
A:
Q: [1] a. What orbitals are used to form the indicated bonds in a-sinensal? (3] -[2] :O: b. In what…
A: a) The orbitals involved in the formation are as follows: [1] σ orbital of sp2 hybridized C of one…
Q: 6. For the following compound, (1) draw the complete Lewis structure; (2) site down the C3- C4 bond…
A: The given compound is: (1) Draw the complete Lewis structure. (2) Site down the C3-C4 bond axis and…
Q: Draw a circle the compound below with the strongest C-X bond and draw a rectangle around the…
A:
Q: The following are 2s, 2p, sp, sp?, and sp orbitals, all drawn to scale and in no particular order.…
A: The given orbitals are 2s,2p,sp,sp2,and sp3 To find: The orbital which is used by the carbon in C-O…
Q: Citric acid is responsible for the tartness of citrus fruits, especially lemons and limes. [3] a.…
A: Since you have posted a question with multiple sub parts, we will solve first three sub parts for…
Q: he structure of 1,2-propadiene (allene) is shown to the right. Q.)Explain the three-dimensional…
A: Structure of 1,2-propadiene is given as,
Q: (1) The two constitutional isomers below have very different boiling points. One has a boiling point…
A: The factors which affect boiling point are: 1. The boiling point increases with increase in the…
Q: 4. A compound has two carbons each with the same set of four different substituents, but does not…
A: The carbon that is attached with the four different substituents is termed as chiral carbon or…
Q: 1) Draw the resonance structures A, B, AND C below. 1) A B + H3C
A: Complete delocalization of pie electron, in a system , it is a interaction of lone pair and pie…
Q: H F 4 3 D F
A: Anti confirmation is more stable than gauche and eclipsed form of Newman projection. Stability…
Q: An sp3 hybridized C − Cl bond is more polar than an sp2 hybridized C − Cl bond. (a) Explain why this…
A: a). The polarity in bond arises due to electronegativity difference between bonded atoms. The…
Q: The purine heterocycle occurs commonly in the structure of DNA. In what type of orbital does each…
A:
Q: What effect does conjugation have on LUMO shape and energy? Table 3. Analysis of LUMO orbitals.…
A:
Q: Identify each illustration as depicting a σ or π bond:(a) side-by-side overlap of a 4p and a 2p…
A: According to valence bond theory (VBT), a covalent bond is formed by overlapping of the half filled…
Q: Explain what interactions occur in the CH3Cl, I2, NH3, and KBr molecules! b. Sort the boiling point…
A: Molecular interactions Strong interactions : ionic bond and covlent bond Weak interactions: 1.…
Q: 2. Lone pair occupies a hybridized p-orbital allowing it to delocalization to a nearby side-way…
A:
Q: D. Cyclopropene structure 39. Why is ethanal incapable of forming a hydrogen bond with another…
A:
Q: 3. The hybridization (sp, sp2..) around each carbon in ethene is and the bond angles around each…
A: The valancy ofcarbon is 4, so there will be 4 bonds around the carbon atom. If the all 4 bonds are…
Q: Classifying a carbon atom by the number of carbons to which it is bonded can also be done in more…
A: Primary carbon (1o): Carbon is attached to only one carbon for example e,g (see above). Secondary…
Q: According to the Newman Projection provided below, the correct dihedral angle is: R₁ H Ca O₁ phi =…
A: As per bartleby guidelines, I'm allowed to answer only first question and it's 3 subparts. please…
Q: В D E
A: from the given compound, only one compound is aromatic which is compound A.
Q: 2. The drug 3,4-methylenedioxymethamphetamine, also known as MDMA or ecstasy, can be synthesized…
A: (a) Here, the 1st carbon is sp2 hybridised (bonded to 3 other carbon atom) and 2nd carbon is sp3…
Q: 2. Show the Atomic and molecular orbitals of acetic acid. Draw the energy diagram for the AO and MO.…
A: To show: atomic and molecular orbitals of acetic acid.
Q: 6. Phomallenic acid C (shown) has antibacterial activity about 20-fold better than other drugs…
A: Let us answer the question in parts. Since there are 4 parts, we will answer only the first three…
Q: 5.2 Consider the compound below. HN- ocellapyridone (a) What orbitals are used to form the indicated…
A: a) The carbon–carbon single bond is a sigma bond and is formed between one hybridized orbital from…
Q: Consider 1-bromopropane, CH3CH2CH2Br. (a) Draw a Newman projection for the conformation in which…
A: To draw a Newman projection first we have to draw a circle for the rear carbon and and put a dot in…
Q: Phosgene, Cl2C = O, has a lower dipole moment than formaldehyde H2C = O. Explain why.
A: The dipole moment is a measure of the polarity within a molecule and is the product of magnitude of…
Q: Propose structures for hydrocarbon molecules that meet the following descriptions: (2 pts. each) (a)…
A:
Q: _8. How many electrons are in the following molecule's largest conjugated a system? a. Two b. Four…
A: A conjugated system is a system of connected p-orbitals with delocalized electrons in compounds…
Q: 2. Draw the Newman projection of the following compounds about the indicated bonds: CH3 H F, CH3 H…
A:
Q: 2. Using the Newman projection shown below as the starting point, with a 0° dihedral angle between…
A: The arrangement of dihedral angle between the front bond and the back bond is 00 is known as…
Q: 3. Draw the Newman projection of the molecule below. Then show the Newman projection for the chair…
A: Concept: Newman projection formula is the one of the stereochemical technique to write a molecule in…
Q: According to the Newman Projection provided below, the correct dihedral angle is: R₁ H Ca O₁ C C phi…
A:
Q: How can draw 3D representation with bond and molecular dipoles? O3 BF3 CO3^2-
A: As per rule only three subparts can be answered. A dipole originates from the more electropositive…
Q: The pentadienyl radical, H2C“CH¬CH“CH¬CH2#, has its unpaired electron delocalized over three carbon…
A: Hey since you have posted a question with multiple sub-parts, we will solve first three sub-parts…
Q: 2) Application of the polygon-and-circle technique (Frost circle) reveals that single electrons…
A:
Q: In
A: In a tabular format, enumerate 5 molecules where the following atoms are found in: O…
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

- Draw the molecular orbital diagram of a linear C-H (i.e. one carbon atom bonded to one hydrogen atom). Draw out the MOs for orbitals containing electrons. 1. Would you expect C-H to behave as a carbocation, carbanion, or radical? 2. Would removal of one electron from C-H to generate C-H+ increase or decrease the bond length between carbon and hydrogen?What are all of the types of orbital overlaps that occur in the above structure. p-p overlap sp²-sp overlap s-sp² overlap sp²-sp² overlap s-sp overlap sp-sp overlap s-s overlap ---------- In cumulene, what are the C=C=C and H−C−H ideal bond angles, respectively?Enter the C=C=C bond angle followed by the H−C−H bond angle separated by a comma (no spaces, no º symbol required).The pentadienyl radical, H2C“CH¬CH“CH¬CH2#, has its unpaired electron delocalized over three carbon atoms.(a) Use resonance forms to show which three carbon atoms bear the unpaired electron.(b) How many MOs are there in the molecular orbital picture of the pentadienyl radical?(c) How many nodes are there in the lowest-energy MO of the pentadienyl system? How many in the highest-energy MO?(d) Draw the MOs of the pentadienyl system in order of increasing energy. (continued)762 CHAPTER 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy(e) Show how many electrons are in each MO for the pentadienyl radical (ground state).(f) Show how your molecular orbital picture agrees with the resonance picture showing delocalization of the unpairedelectron onto three carbon atoms.(g) Remove the highest-energy electron from the pentadienyl radical to give the pentadienyl cation. Which carbon atomsshare the positive charge? Does this picture agree with the resonance picture?(h) Add an…
- The pentadienyl radical, H2C“CH¬CH“CH¬CH2#, has its unpaired electron delocalized over three carbon atoms.(a) Use resonance forms to show which three carbon atoms bear the unpaired electron.(b) How many MOs are there in the molecular orbital picture of the pentadienyl radical?(c) How many nodes are there in the lowest-energy MO of the pentadienyl system? How many in the highest-energy MO?(d) Draw the MOs of the pentadienyl system in order of increasing energy1. Explain the two ways that methane (CH4) illustrates the need for hybridized molecular bonds. Draw a picture to represent the hybridization of carbon's orbitals and their overlap with hydrogen's. Label each orbital used in bonding with its letters and each bond as sigma or pi.What are the overlapping orbitals between nitrogen and carbon in aniline? sp2-sp2 p-p sp3-sp3 sp3-sp2
- (a) How many π molecular orbitals are present in deca-1,3,5,7,9-pentaene (CH2 = CH – OH = OH – CH = CH – OH = OH – CH = CH2)? (b) How many are bonding MOs and how many are antibonding MOs? (c) How many nodes are present in ψ1? (d) How many nodes are present in ψ10*Let us construct the molecular orbital diagram of ethylene (in pieces). a. First, construct the MO diagram of linear carbene (CH2). Draw pictures of all 6 orbitals b. Now bend the carbene to a bond angle of about 120°. How does this change your MO diagram? Draw pictures of all 6 orbitals. c. Now bring two of these carbene molecules together to make ethylene. Draw pictures of all 12 orbitals.Consider the compound in Figure 3. Which types of orbitals overlap to make the bond indicated in red? * A- sp³ – sp³ B- sp² – sp² C- sp³ – sp² D- sp² – sp³ E- None of these options is correct
- he structure of 1,2-propadiene (allene) is shown to the right. Q.)State the orbital hybridization of each carbon.In a tabular format, enumerate 5 molecules where the following atoms are found in: O (sp3-hybridized) Oxygen (sp2-hybridized) Nitrogen (sp2-hybridized) Carbon (sp2-hybridized)Is the geometry of CH2BrCl, the same as CH3Cl and CH4? Briefly explain the evidence for your answer

