6.) The diagram below shows the deposition of iodine vapor at room temperature. Which statement is correct? 00 A. AH<0; AS < 0, AG < 0 B. AH > 0, AS > 0, AG <0 C. AH > 0, AS > 0, AG > 0 D. AH < 0, AS <0, AG > 0 Keywords: endothermic, exothermic, entropy, entropy change, spontaneous, heat flow, attractive force, condense phase, nonspontaneous Concepts: Gibbs Free Energy Change
6.) The diagram below shows the deposition of iodine vapor at room temperature. Which statement is correct? 00 A. AH<0; AS < 0, AG < 0 B. AH > 0, AS > 0, AG <0 C. AH > 0, AS > 0, AG > 0 D. AH < 0, AS <0, AG > 0 Keywords: endothermic, exothermic, entropy, entropy change, spontaneous, heat flow, attractive force, condense phase, nonspontaneous Concepts: Gibbs Free Energy Change
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 126CP: You have two distinct gaseous compounds made from element X and element Y. The mass percents are as...
Related questions
Question
100%
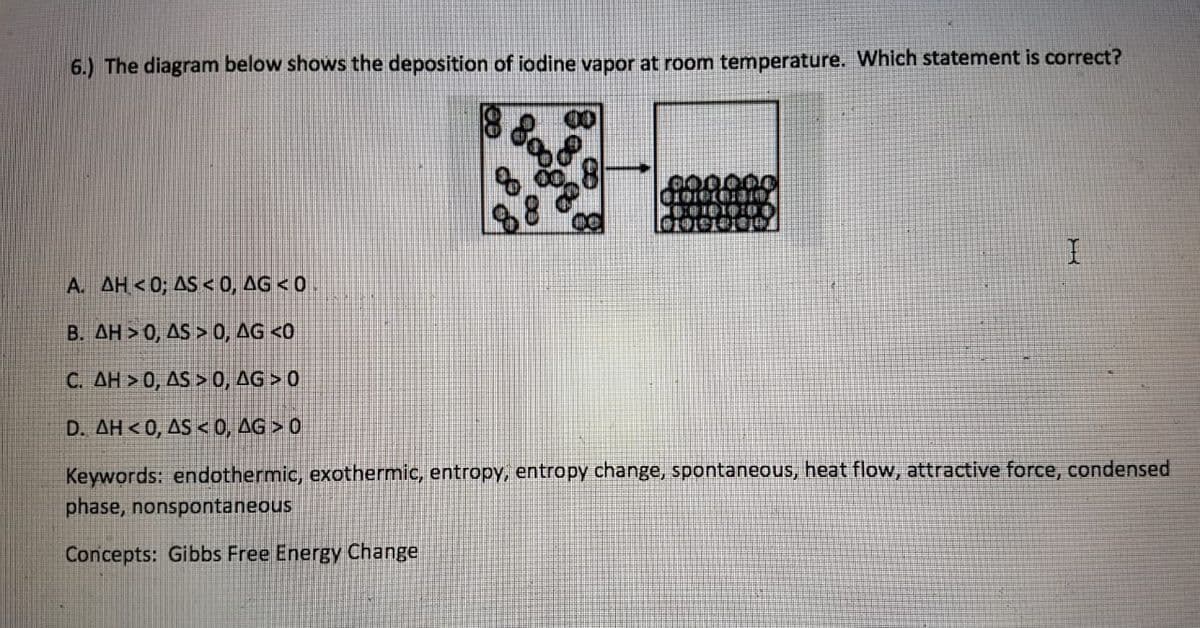
Transcribed Image Text:6.) The diagram below shows the deposition of iodine vapor at room temperature. Which statement is correct?
88
D:11中:0串
A. AH<0; AS <0, AG <0
B. AH > 0, AS > 0, AG <0
C. AH > 0, AS > 0, AG > 0
D. AH < 0, AS < 0, AG > 0
Keywords: endothermic, exothermic, entropy, entropy change, spontaneous, heat flow, attractive force, condensed
phase, nonspontaneous
Concepts: Gibbs Free Energy Change
Expert Solution
Step 1
The ordered system is the indication of low entropy. While less orderliness is the indication of an increase in the entropy level of the system. If the product has more entropy than the reactant the entropy is negative. If the product has less entropy than the reactant the entropy is positive.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning