6. The following table shows the first ionization energy for elements A, B and C. Element 114 12B 14C First ionization energy (kJ/mol) 496 738 1000 35 30 Based on the above table. p35 (a) Write the electronic configuration of A, B and C. (b) Give the reasons why the first ionization energy increase from A to C. Bloarst in te Some perad s aerry tu red hen
6. The following table shows the first ionization energy for elements A, B and C. Element 114 12B 14C First ionization energy (kJ/mol) 496 738 1000 35 30 Based on the above table. p35 (a) Write the electronic configuration of A, B and C. (b) Give the reasons why the first ionization energy increase from A to C. Bloarst in te Some perad s aerry tu red hen
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 109E
Related questions
Question
How to do it
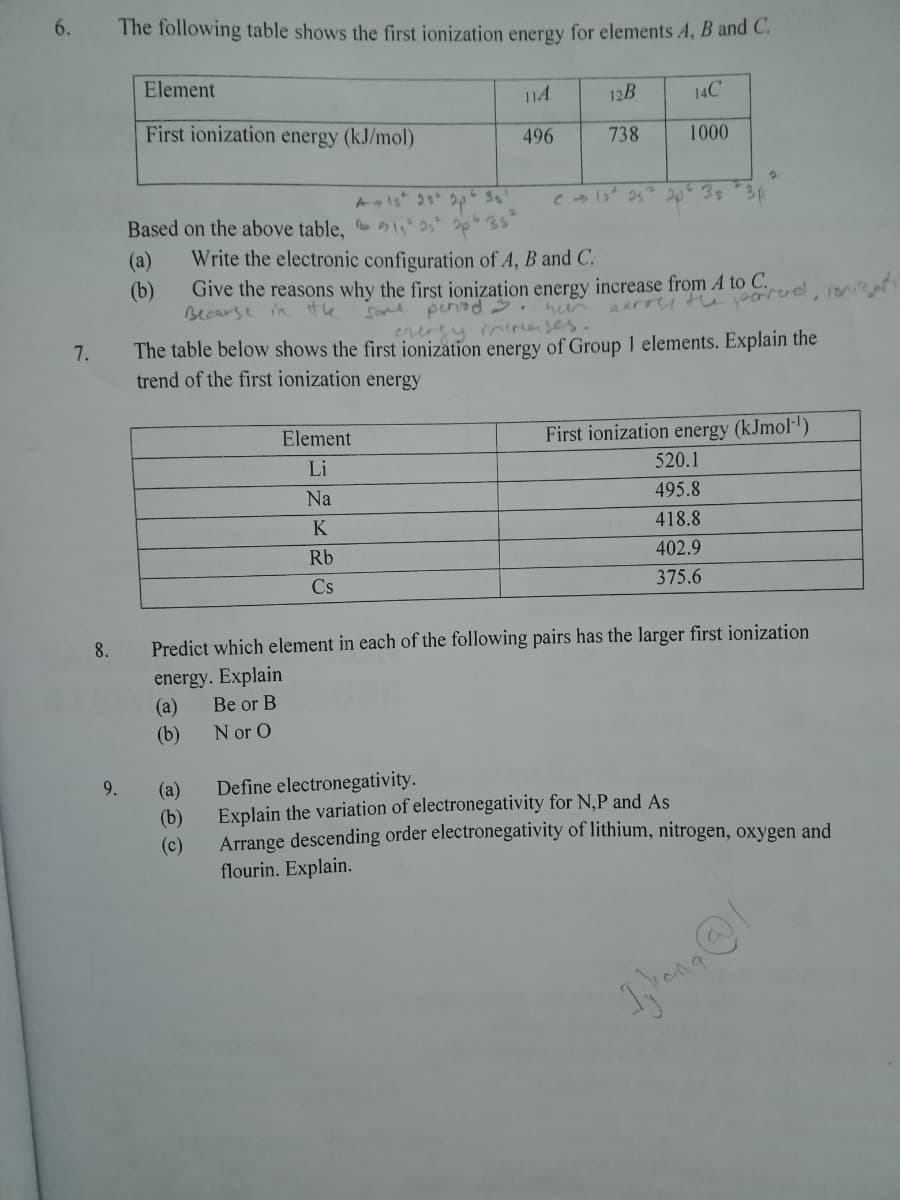
Transcribed Image Text:6.
The following table shows the first ionization energy for elements A, B and C.
Element
114
12B
14C
First ionization energy (kJ/mol)
496
738
1000
2035
3p
Based on the above table, 42 2p 35
(а)
(b)
Write the electronic configuration of A, B and C.
Give the reasons why the first ionization energy increase from A to C.
Becarse in
the
Some penad.
aerry tu arud, ne
enerey inerenses.
The table below shows the first ionization energy of Group 1 elements. Explain the
7.
trend of the first ionization energy
Element
First ionization energy (kJmol-')
Li
520.1
Na
495.8
K
418.8
Rb
402.9
Cs
375.6
8.
Predict which element in each of the following pairs has the larger first ionization
energy. Explain
(а)
(b)
Be or B
N or O
9.
(а)
Define electronegativity.
(b)
Explain the variation of electronegativity for N,P and As
Arrange descending order electronegativity of lithium, nitrogen, oxygen and
flourin. Explain.
(c)
and
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning