When the ionization energies (IE) of a series of isoelectronic atoms and ions in the second ow (that is, species that contain the same number of electrons, in this case three) are compared, the following relationship is observed (IE are in kJ/mol, and Z is the atomic number):| VIE = 18.4Z – 32.0 What is the expected ionization energy for Be*? A) 1730 kJ/mol 3) 23.2 kJ/mol C) 538 kJ/mol D) 41.6 kJ/mol E) 6.4 kJ/mol
When the ionization energies (IE) of a series of isoelectronic atoms and ions in the second ow (that is, species that contain the same number of electrons, in this case three) are compared, the following relationship is observed (IE are in kJ/mol, and Z is the atomic number):| VIE = 18.4Z – 32.0 What is the expected ionization energy for Be*? A) 1730 kJ/mol 3) 23.2 kJ/mol C) 538 kJ/mol D) 41.6 kJ/mol E) 6.4 kJ/mol
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 39P: Without consulting any tables, arrange the following substances in order and explain your choice of...
Related questions
Question
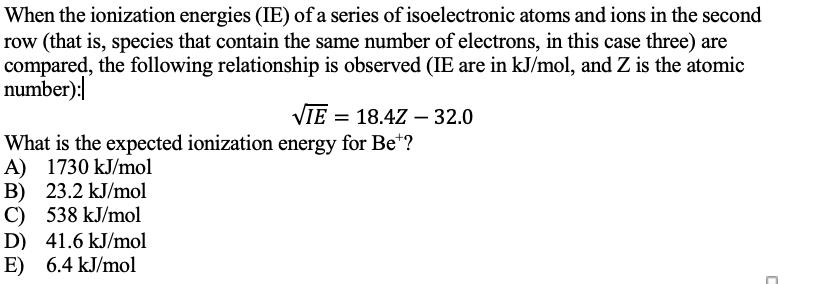
Transcribed Image Text:When the ionization energies (IE) of a series of isoelectronic atoms and ions in the second
row (that is, species that contain the same number of electrons, in this case three) are
compared, the following relationship is observed (IE are in kJ/mol, and Z is the atomic
number):
VIE = 18.4Z - 32.0
What is the expected ionization energy for Be"?
A) 1730 kJ/mol
B) 23.2 kJ/mol
C) 538 kJ/mol
D) 41.6 kJ/mol
E) 6.4 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning