Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter37: Qualitative Analysis Of Group Ii Cations
Section: Chapter Questions
Problem 3ASA
Related questions
Question
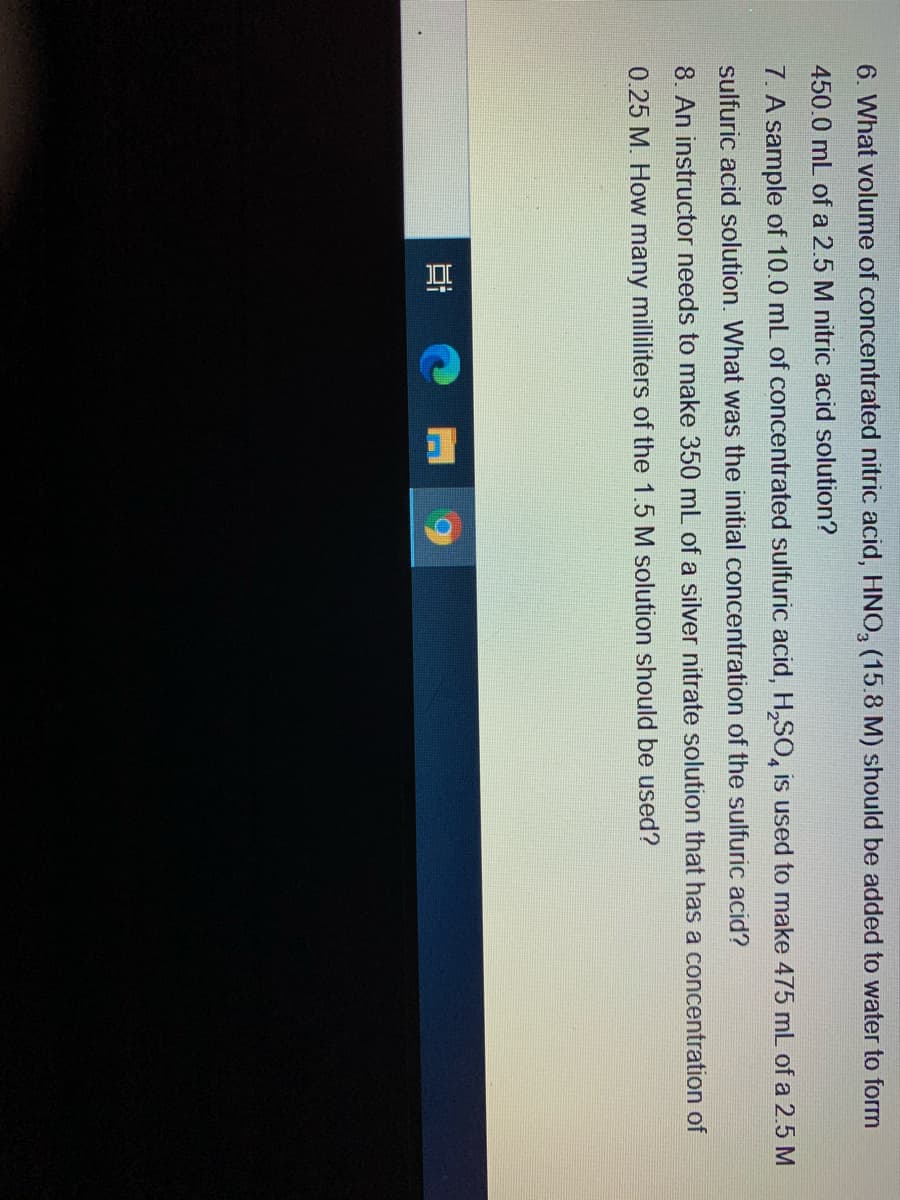
Transcribed Image Text:6. What volume of concentrated nitric acid, HNO, (15.8 M) should be added to water to form
450.0 mL of a 2.5 M nitric acid solution?
7. A sample of 10.0 mL of concentrated sulfuric acid, H,SO, is used to make 475 mL of a 2.5 M
sulfuric acid solution. What was the initial concentration of the sulfuric acid?
8. An instructor needs to make 350 mL of a silver nitrate solution that has a concentration of
0.25 M. How many milliliters of the 1.5 M solution should be used?
耳
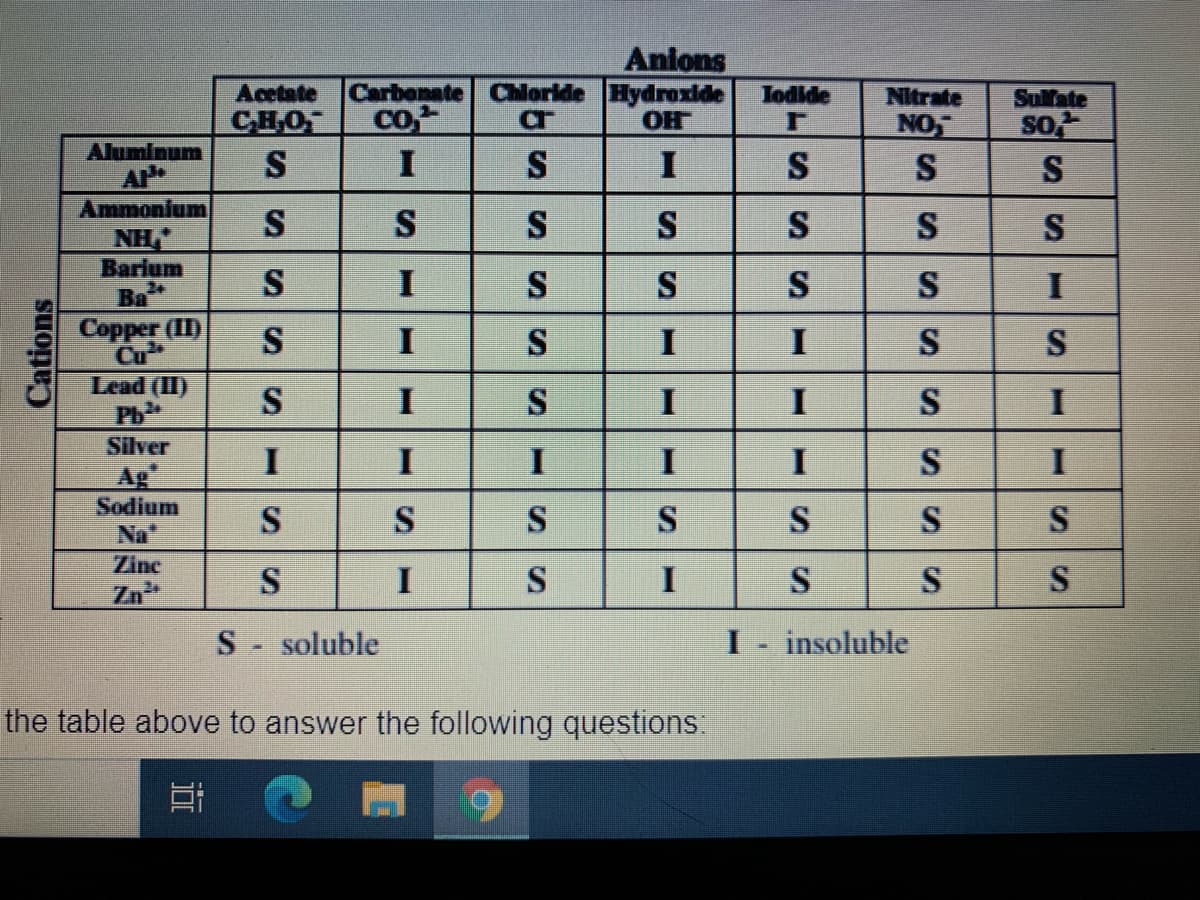
Transcribed Image Text:Acetate
CHO
S
Anions
Carbonate Chloride Hydroxide
OH
Jodide
co,
Nitrate
NO
Sulfate
so
Aluminum
I
S
I
S
S
S
Ammonium
NH,"
Barium
Ba
Copper (II)
Cu
Lead (II)
Ph
S
S
S
S
S
S
S
S
I
S
S
S
I
S
S
S
S
I
S
Silver
Ag
Sodium
Na"
I
I
S
S
S
S
Zinc
S
S
Zn
S- soluble
insoluble
the table above to answer the following questions:
Cations
SI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole
