Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter11: Organic Compounds: Alkanes
Section: Chapter Questions
Problem 11.10E
Related questions
Question
If 25.0 mL of a 6.00 M HCL solution is diluted to 2.00 L, what is the molarity of the new solution?
Determine the volume (in mL) of water that needs to be added to 25.0 mL of a 0.250 M NaBr to produce a 0.0275 M solution. Assume the volumes are additive.
![Question 22 of 30
Submit
Provide the correct systematic
name for the compound shown
here.
2- N- 4- N,N- 3- 5-
tetra di tri
prop meth but hex eth pent
oic acid yl amide amine] an al
ZI](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc0da3887-c4ea-4b9e-9efc-f9c8b5a90f3a%2Ff49a4b27-c327-430c-82e9-0d35b6b69613%2Fv4noyzb_processed.png&w=3840&q=75)
Transcribed Image Text:Question 22 of 30
Submit
Provide the correct systematic
name for the compound shown
here.
2- N- 4- N,N- 3- 5-
tetra di tri
prop meth but hex eth pent
oic acid yl amide amine] an al
ZI
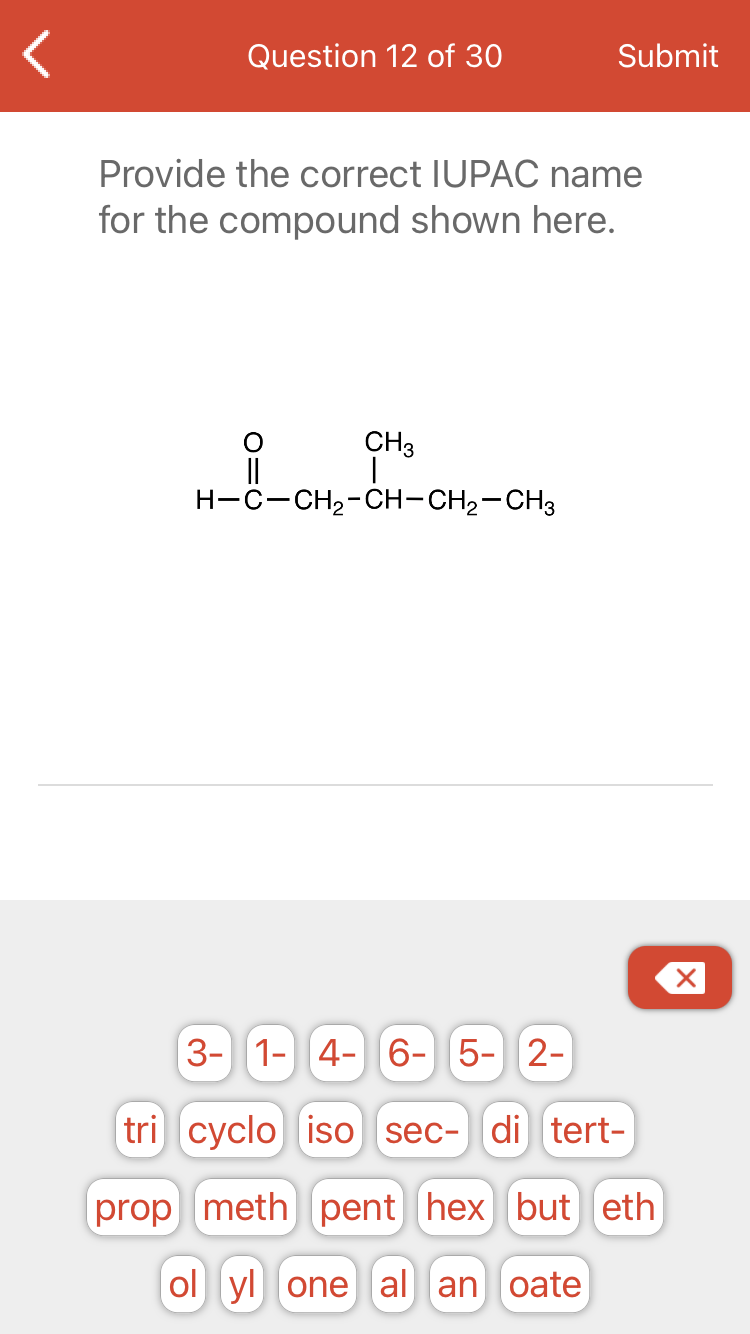
Transcribed Image Text:Question 12 of 30
Submit
Provide the correct IUPAC name
for the compound shown here.
CH3
||
H-C-CH2-CH-CH2-CH3
3- 1- 4- 6-5-||2-
tri cyclo iso sec- di tert-
prop meth pent hex but eth
ol yl one al an oate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning