6.125 A 175-g pipe is heated to 78.24°C. The pipe is then placed at transferred out of the water, what is the specific heat of the in 100.0 g of water held in a calorimeter at 25.00°C. The final temperature of the mixture is 33.43°C. The specific heat of water is 4.184 J/(g °C). Assuming no heat is pipe? Using Table 6.2, determine what the pipe could he made of.
6.125 A 175-g pipe is heated to 78.24°C. The pipe is then placed at transferred out of the water, what is the specific heat of the in 100.0 g of water held in a calorimeter at 25.00°C. The final temperature of the mixture is 33.43°C. The specific heat of water is 4.184 J/(g °C). Assuming no heat is pipe? Using Table 6.2, determine what the pipe could he made of.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 12E: An aluminum kettle weighs 1.05 kg. (a) What is the heat capacity of the kettle? (b) How much heat is...
Related questions
Question
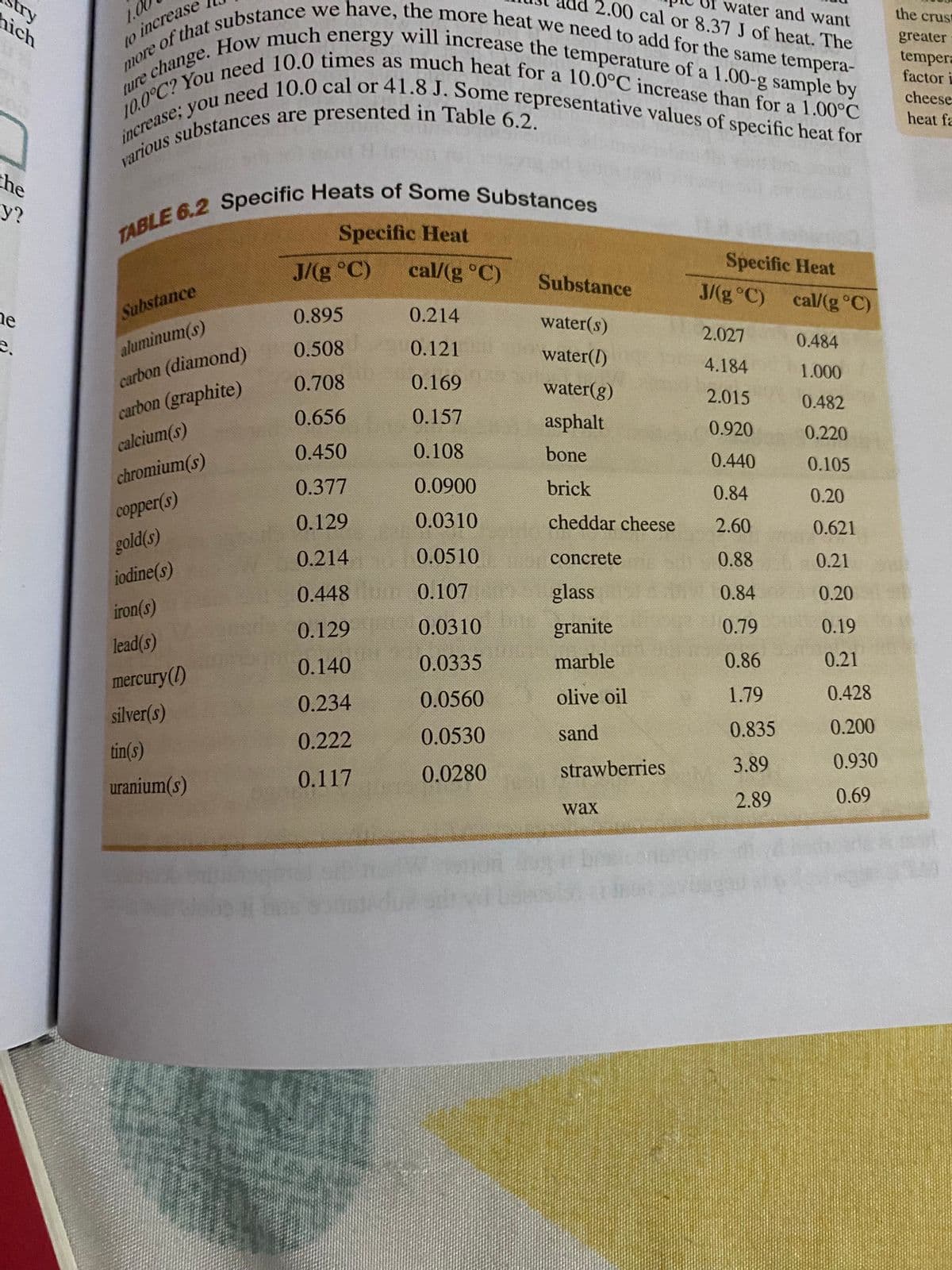
Transcribed Image Text:increase; you need 10.0 cal or 41.8 J. Some representative values of specific heat for
10.0°C? You need 10.0 times as much heat for a 10.0°C increase than for a 1.00°C
e change. How much energy will increase the temperature of a 1.00-g sample by
e of that substance we have, the more heat we need to add for the same tempera-
nich
try
ad 2.00 cal or 8.37 J of heat. The
water and want
10
the crust
to increase
greater
tempera
factor i
cheese
a
heat fa
the
y?
Specific Heat
Specific Heat
J/(g °C)
cal/(g °C)
Substance
J/(g °C)
cal/(g °C)
Substance
0.214
0.895
ne
e.
water(s)
2.027
0.484
aluminum(s)
0.121
0.508
water(I)
4.184
1.000
carbon (diamond)
0.169
0.708
water(g)
2.015
0.482
carbon (graphite)
0.157
0.656
asphalt
0.920
0.220
calcium(s)
0.108
0.450
bone
0.440
0.105
chromium(s)
0.0900
0.377
brick
0.84
0.20
соpper(s)
0.0310
0.129
cheddar cheese
2.60
0.621
gold(s)
0.214
0.0510
0o concrete
0.88
0.21
iodine(s)
0.448 0.107
glass
0.84
0.20
iron(s)
0.129
0.0310
granite
0.79
0.19
lead(s)
0.140
0.0335
marble
0.86
0.21
mercury(1)
0.234
0.0560
olive oil
1.79
0.428
silver(s)
0.0530
sand
0.835
0.200
0.222
tin(s)
strawberries
3.89
0.930
0.117
0.0280
uranium(s)
2.89
0.69
wax
Transcribed Image Text:at transferred out of the water, what is the specific heat of the
final temperature of the mixture is 33.43°C. The specific
6.125 A 175-g pipe is heated to 78.24°C. The pipe is then placed
in 100.0 g of water held in a calorimeter at 25.00°C. The
ct with
heat of water is 4.184 J/(g °C). Assuming no heat is
transferred out of the water, what is the specific heat of th
s the
pipe? Using Table 6.2, determine what the pipe conld be
to
made of.
sl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
