A.2. Basic ndition eno liza fion choose the correct arr a / reversible LDA arirreversible: comjugate fo base pka =17 LOA : resmance form Lich comverted to lithimm alisopropylamide pka= 96 mehoose the corre ct arr od c rewersible , or irrevergible :) * BuOk し comjugate base resmance pka = 17 form その :0-C-CM3 comvented H-0-C-CH3 to
A.2. Basic ndition eno liza fion choose the correct arr a / reversible LDA arirreversible: comjugate fo base pka =17 LOA : resmance form Lich comverted to lithimm alisopropylamide pka= 96 mehoose the corre ct arr od c rewersible , or irrevergible :) * BuOk し comjugate base resmance pka = 17 form その :0-C-CM3 comvented H-0-C-CH3 to
Chapter20: Carboxylic Acids And Nitriles
Section20.SE: Something Extra
Problem 20VC: Electrostatic potential maps of anisole and thioanisole are shown. Which do you think is the...
Related questions
Question
100%
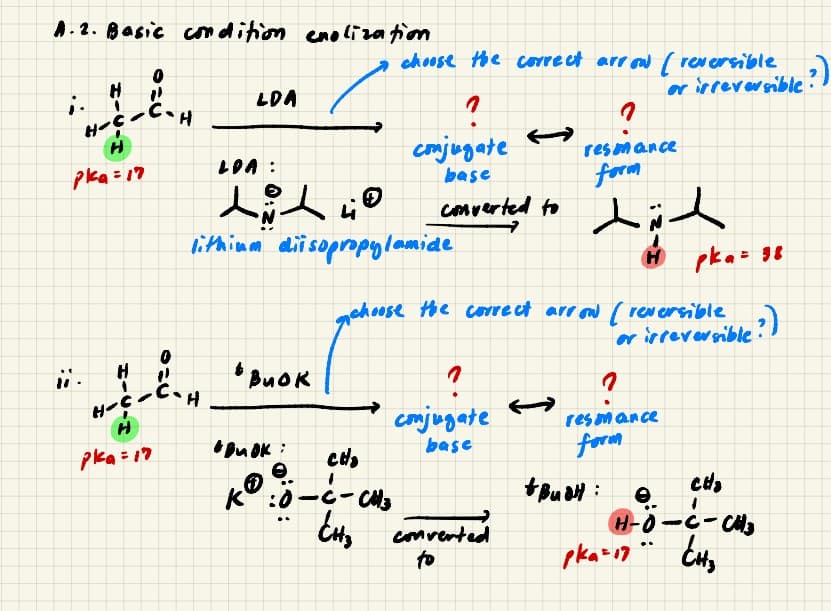
Transcribed Image Text:A.2. Basic comdition enoliza fiom
choose the covre ct arr ow ( reversible
ar irreverible?)
LDA
comjugate
resmance
LOA :
pka=17
form
base
dich
comverted to
lithimm disopropylamide
H pka= 98
choose the correct arr ow ( reversible
or irreversible?
H
* BuOk
H.
comjugate
resmance
pka = 17
base
form
e.
K°:0
EH,
comverted
..
to

Transcribed Image Text:A. 2. The a - hydrogen has a
it conled undergo Bransted acid - base reactm with
strong bases and conjugate base of acids to form
enslate and enst, respectively. Show the arrow
pushing mechani'sm af enolization in the examples
provided belomo:
pka ef around 17-20 . Thus,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT