7-11. Limestone consists mainly of the mineral calcite, CaCO3. The carbonate content of 0.541 3 g of powdered limestone was measured by suspending the powder in water, adding 10.00 mL of 1.396 M HCl, and heating to dissolve the solid and expel CO2: CACO3 (s) +2H* → Ca²+ +CO,↑ +H2O Calcium carbonate FM 100.086 The excess acid required 39.96 mL of 0.100 4 M NaOH for complete titration to a phenolphthalein end point. Find the weight percent of calcite in the limestone.
7-11. Limestone consists mainly of the mineral calcite, CaCO3. The carbonate content of 0.541 3 g of powdered limestone was measured by suspending the powder in water, adding 10.00 mL of 1.396 M HCl, and heating to dissolve the solid and expel CO2: CACO3 (s) +2H* → Ca²+ +CO,↑ +H2O Calcium carbonate FM 100.086 The excess acid required 39.96 mL of 0.100 4 M NaOH for complete titration to a phenolphthalein end point. Find the weight percent of calcite in the limestone.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
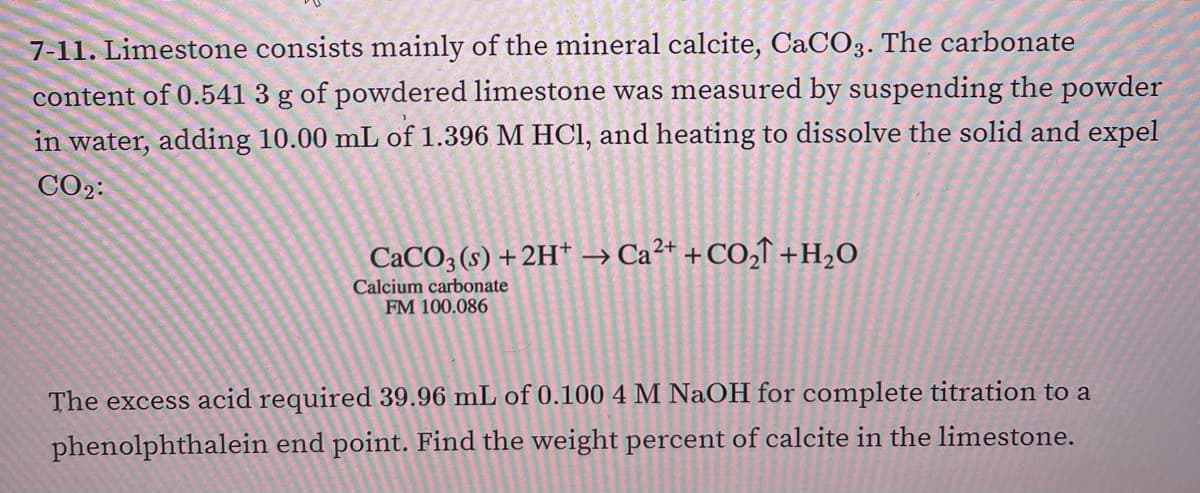
Transcribed Image Text:7-11. Limestone consists mainly of the mineral calcite, CaCO3. The carbonate
content of 0.541 3 g of powdered limestone was measured by suspending the powder
in water, adding 10.00 mL of 1.396 M HCI, and heating to dissolve the solid and expel
CO2:
CACO3 (s) +2H* → Ca²* +CO,T +H2O
Calcium carbonate
FM 100.086
The excess acid required 39.96 mL of 0.100 4 M NaOH for complete titration to a
phenolphthalein end point. Find the weight percent of calcite in the limestone.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
