7) Adding one or more electrons to a neutral atom forms an anion (an ion with negative charge). Removing one or more electrons from an atom forms a cation (an ion with positive charge). Why is the radius of an anion always larger than the neutral atom it was formed from? Explain why is this different than the trend in, for example, the 3p neutral atomic series shown below where electrons are added but the radius decreases. Atoms with increasing number of 3p electrons 0000 s)(c) (Ar Al si )( P S CI
7) Adding one or more electrons to a neutral atom forms an anion (an ion with negative charge). Removing one or more electrons from an atom forms a cation (an ion with positive charge). Why is the radius of an anion always larger than the neutral atom it was formed from? Explain why is this different than the trend in, for example, the 3p neutral atomic series shown below where electrons are added but the radius decreases. Atoms with increasing number of 3p electrons 0000 s)(c) (Ar Al si )( P S CI
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter6: The Periodic Table And Periodic Law
Section: Chapter Questions
Problem 66A
Related questions
Question
Help
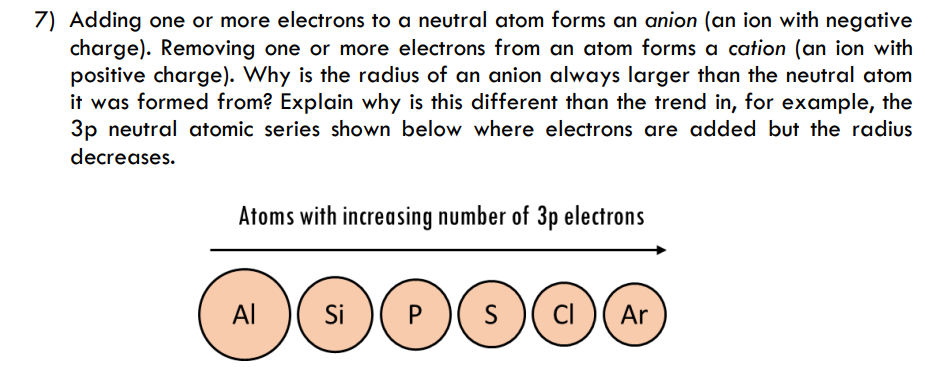
Transcribed Image Text:7) Adding one or more electrons to a neutral atom forms an anion (an ion with negative
charge). Removing one or more electrons from an atom forms a cation (an ion with
positive charge). Why is the radius of an anion always larger than the neutral atom
it was formed from? Explain why is this different than the trend in, for example, the
3p neutral atomic series shown below where electrons are added but the radius
decreases.
Atoms with increasing number of 3p electrons
Al
Si
P
S
s )( ci )( Ar
CI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning