Re-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size. atoms or ions in order of decreasing size atoms or jons CI, Mg. S O.0.0 Rb, Rb. Cs O.0.0 sC1, s 0.0.0
Re-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size. atoms or ions in order of decreasing size atoms or jons CI, Mg. S O.0.0 Rb, Rb. Cs O.0.0 sC1, s 0.0.0
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 34P: For each of the following pairs of atoms or ions, state which you expect to have the larger radius....
Related questions
Question
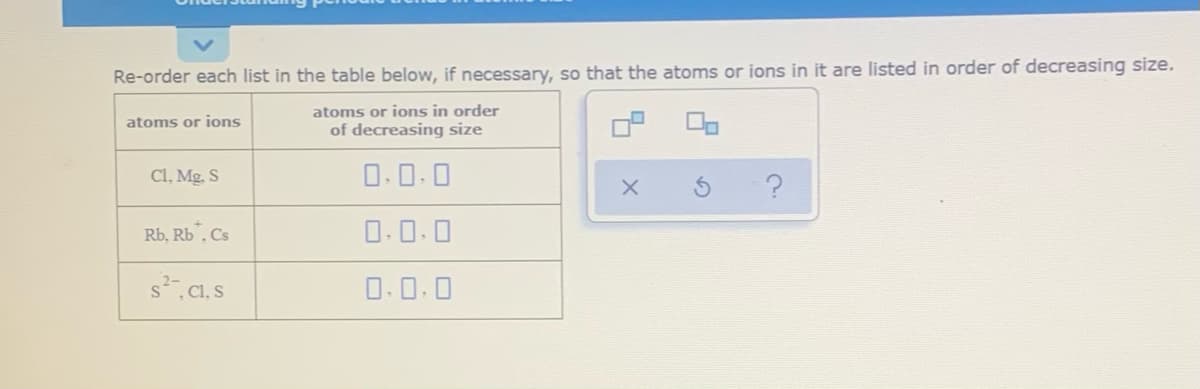
Transcribed Image Text:Re-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size.
atoms or ions in order
of decreasing size
atoms or jons
Cl, Mg. S
O.0.0
Rb, Rb". Cs
O.0.0
s CI, S
O.0.0
Expert Solution
Step 1
Mg > S > Cl
As we move left to right in a period, atomic redius decreases due to effective nuclear charge.
Cs> Rb > Rb+
As we move down the group, number of shell increases consequently size increases.
Cation has small size than corresponding atom due to higher effective nuclear charge .
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
