7) Rank the protons in inčreasing acidity and explain your ranking rationale. 1 3 2>1)3 3 less acidic because inductive ffecf is lass and further away from Co lbends. less acidic than z be cau se the 2nQ H has double, the nductne effect. 8) Consider 1.2-dibromoethene, shown below. Use arrows to represent the individual bond
7) Rank the protons in inčreasing acidity and explain your ranking rationale. 1 3 2>1)3 3 less acidic because inductive ffecf is lass and further away from Co lbends. less acidic than z be cau se the 2nQ H has double, the nductne effect. 8) Consider 1.2-dibromoethene, shown below. Use arrows to represent the individual bond
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 13E: Complete each Lewis structure, draw all important resonance structures, predict a value for thebond...
Related questions
Question
Is my rationale correct?
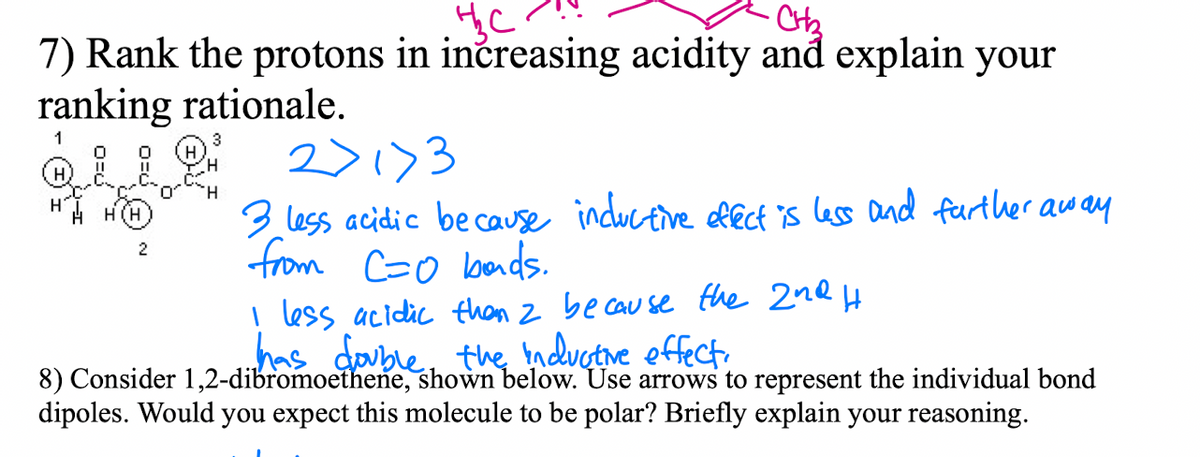
Transcribed Image Text:7) Rank the protons in inčreasing acidity and explain your
ranking rationale.
1
3
2>1)3
3 less acidic be cause inductive lect is less and feurther away
from Co bads.
H.
H(H
2
less acidic than z be cau se fhe 2nQ H
has double, the nduetive effect.
8) Consider 1,2-dibromoethene, shown below. Use arrows to represent the individual bond
dipoles. Would you expect this molecule to be polar? Briefly explain your reasoning.
Expert Solution
Step 1 Part-1
Your order is correct
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
