7) To elements in the gas state react to form a new compound. Based on the state of matter of the reactants, what state of matter should the resulting compound be? A) Gas B) Liquid C) Solid D) The resulting compound doesn't depend on the states of matter of the reactants. 8) A chemistry student reacts a black metallic element with an element that exists as a bright blue powder. What will the resulting chemical compound look like? A) The compound will appear mostly black since the color black will overpower the bright blue color. B) The appearance of the chemical compound is not dependent on the color of the original elements. C) The color of the resulting compound will be a mixture of black and bright blue, leaning mainly towards the color black. D) The color of the resulting compound will be a mixture of black and bright blue, leaning mainly towards the color bright blue.
7) To elements in the gas state react to form a new compound. Based on the state of matter of the reactants, what state of matter should the resulting compound be? A) Gas B) Liquid C) Solid D) The resulting compound doesn't depend on the states of matter of the reactants. 8) A chemistry student reacts a black metallic element with an element that exists as a bright blue powder. What will the resulting chemical compound look like? A) The compound will appear mostly black since the color black will overpower the bright blue color. B) The appearance of the chemical compound is not dependent on the color of the original elements. C) The color of the resulting compound will be a mixture of black and bright blue, leaning mainly towards the color black. D) The color of the resulting compound will be a mixture of black and bright blue, leaning mainly towards the color bright blue.
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 48A
Related questions
Question
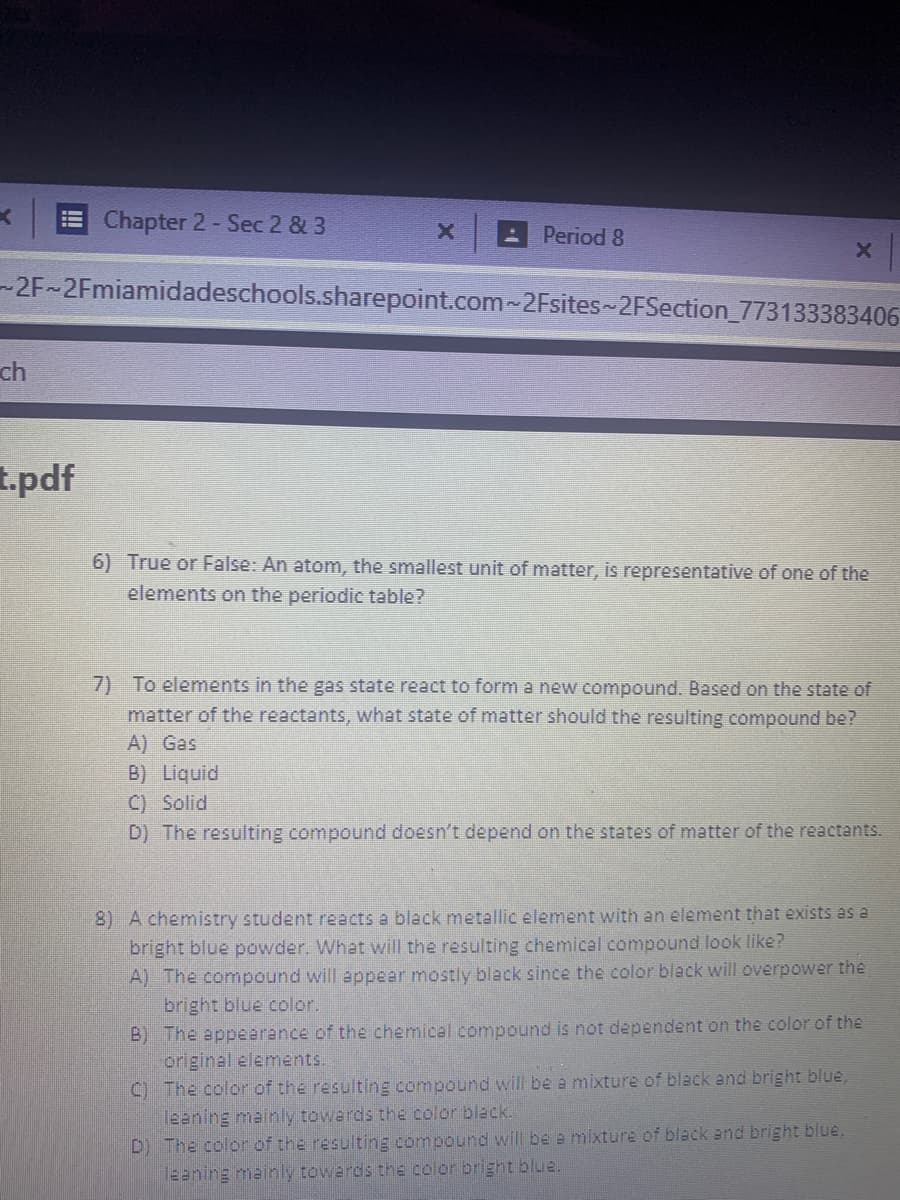
Can you do number 7 or 7 and 8
Transcribed Image Text:Chapter 2- Sec 2 & 3
Period 8
-2F~2Fmiamidadeschools.sharepoint.com~2Fsites-2FSection_773133383406
ch
t.pdf
6) True or False: An atom, the smallest unit of matter, is representative of one of the
elements on the periodic table?
7) To elements in the gas state react to form a new compound. Based on the state of
matter of the reactants, what state of matter should the resulting compound be?
A) Gas
B) Liquid
C) Solid
D) The resulting compound doesn't depend on the states of matter of the reactants.
8) A chemistry student reacts a black metallic element with an element that exists as a
bright blue powder. What will the resulting chemical compound look like?
A) The compound will appear mostly black since the color black will overpower the
bright blue color.
B) The appearance of the chemical compound is not dependent on the color of the
original elements.
C) The color of the resulting compound will be a mixture of black and bright blue,
leaning mainly towards the color black.
D) The color of the resulting compound will be a mixture of black and bright blue,
leening mainly towards the color bright blue.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning