7. Consider the following T-z-y and P-z-y diagrams for the binary system methanol-ethlyl acetate (Murti, P., & Van Winkle, M. (1958) Industrial & Engineering Chemistry Chemical and Engineering Data Series 3.1 (1958): 72-81) TURE C 75
7. Consider the following T-z-y and P-z-y diagrams for the binary system methanol-ethlyl acetate (Murti, P., & Van Winkle, M. (1958) Industrial & Engineering Chemistry Chemical and Engineering Data Series 3.1 (1958): 72-81) TURE C 75
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.55E
Related questions
Question
Pleee help, I will upvote
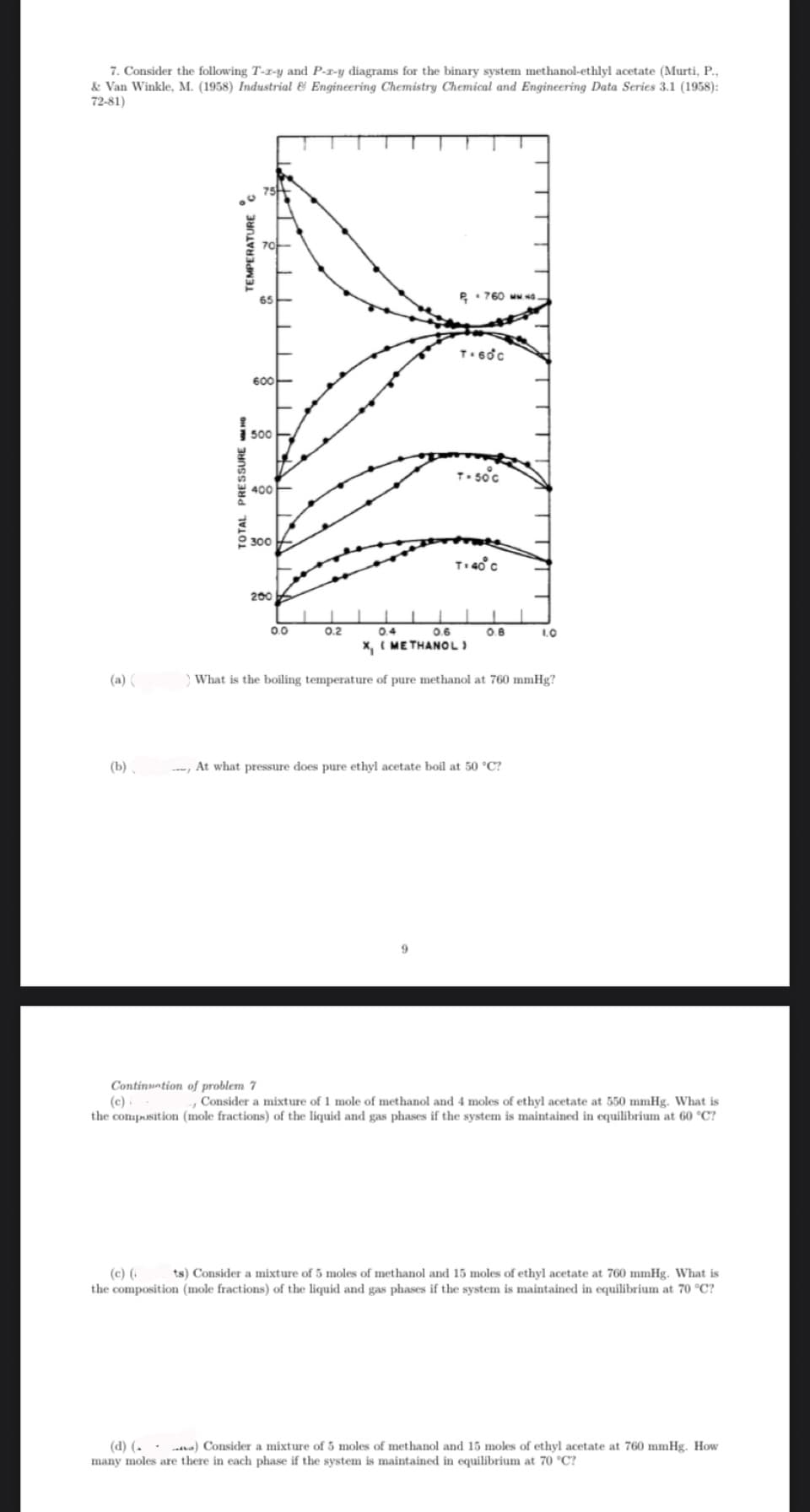
Transcribed Image Text:7. Consider the following T-z-y and P-r-y diagrams for the binary system methanol-ethlyl acetate (Murti, P.,
& Van Winkle, M. (1958) Industrial & Engineering Chemistry Chemical and Engineering Data Series 3.1 (1958):
72-81)
(a) (
(b)
TEMPERATURE C
TOTAL PRESSURE MMHG
600
500
400
300
200
0.0
0.2
760 MM.
T. 60c
T-50°C
0.4
0.6
X, (METHANOL
T: 40° C
0.8
1
- At what pressure does pure ethyl acetate boil at 50 °C?
1.0
) What is the boiling temperature of pure methanol at 760 mmHg?
Continuation of problem 7
(c)
- Consider a mixture of 1 mole of methanol and 4 moles of ethyl acetate at 550 mmHg. What is
the composition (mole fractions) of the liquid and gas phases if the system is maintained in equilibrium at 60 °C?
(c) ( ts) Consider a mixture of 5 moles of methanol and 15 moles of ethyl acetate at 760 mmHg. What is
the composition (mole fractions) of the liquid and gas phases if the system is maintained in equilibrium at 70 °C?
(d) (.. .) Consider a mixture of 5 moles of methanol and 15 moles of ethyl acetate at 760 mmHg. How
many moles are there in each phase if the system is maintained in equilibrium at 70 °C?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
