7. For each of these, draw the two conformers by adding the missing methyl and ethyl group. The second one should be the ring flip of the first. Identify which is more stable. As we learned, the larger group prefers the equatorial position. Me ring Et flip ring Me. Et flip ring Et flip Me
7. For each of these, draw the two conformers by adding the missing methyl and ethyl group. The second one should be the ring flip of the first. Identify which is more stable. As we learned, the larger group prefers the equatorial position. Me ring Et flip ring Me. Et flip ring Et flip Me
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter2: Alkanes And Cycloalkanes
Section2.5: Conformations Of Alkanes And Cycloalkanes
Problem 2.9P: Following is a chair conformation of cyclohexane with the carbon atoms numbered 1 through 6. (a)...
Related questions
Question
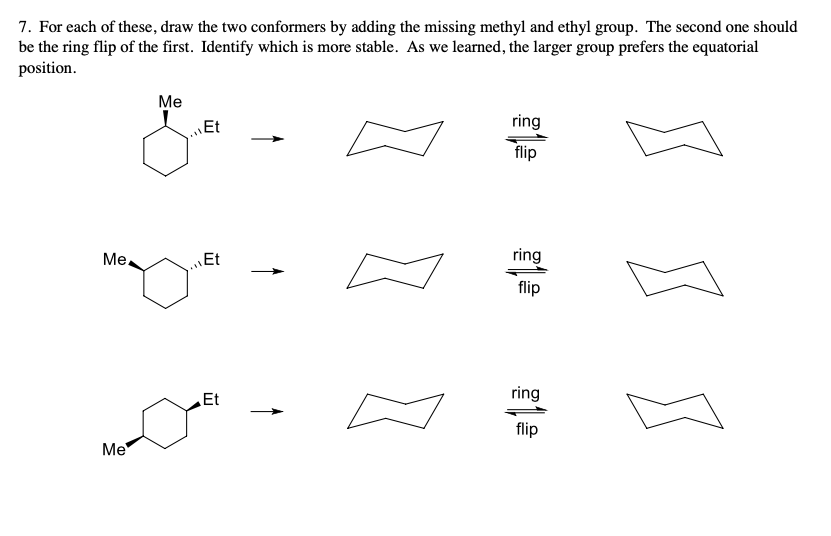
Transcribed Image Text:7. For each of these, draw the two conformers by adding the missing methyl and ethyl group. The second one should
be the ring flip of the first. Identify which is more stable. As we learned, the larger group prefers the equatorial
position.
Me
ring
Et
flip
ring
Me.
Et
flip
ring
Et
flip
Me
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning