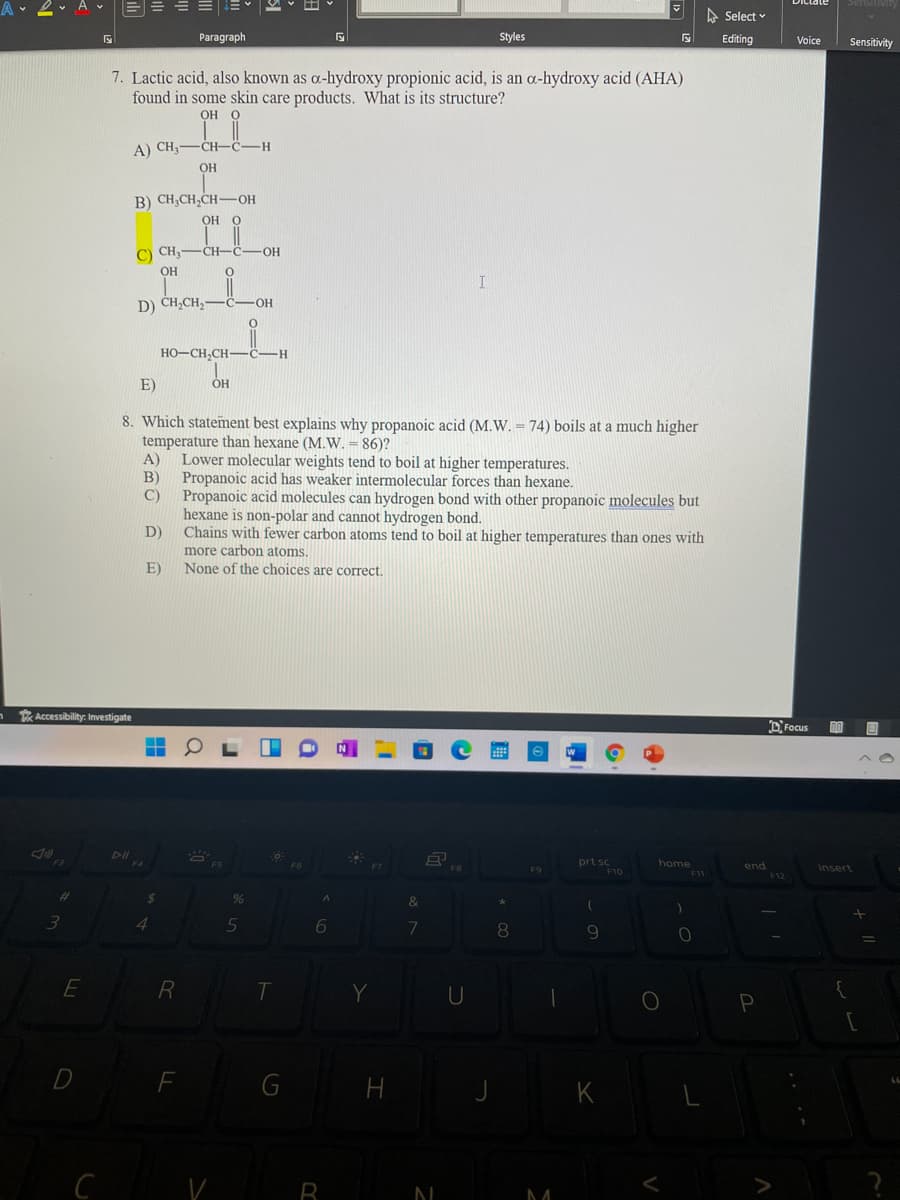
7. Lactic acid, also known as a-hydroxy propionic acid, is an a-hydroxy acid (AHA) found in some skin care products. What is its structure? OH O A) CH,-CH-ċ-H OH B) CH,CH,CH-OH OH O C) CHy OH -CH-C- HO- D) CH,CH, HO-CH,CH-C- H- E) OH 8. Which statement best explains why propanoic acid (M.W. = 74) boils at a much higher temperature than hexane (M.W. = 86)? A) Lower molecular weights tend to boil at higher temperatures. Propanoic acid has weaker intermolecular forces than hexane. Propanoic acid molecules can hydrogen bond with other propanoic molecules but hexane is non-polar and cannot hydrogen bond. D) B) Chains with fewer carbon atoms tend to boil at higher temperatures than ones with more carbon atoms. None of the choices are correct. E) wility: Investigate DFocus
7. Lactic acid, also known as a-hydroxy propionic acid, is an a-hydroxy acid (AHA) found in some skin care products. What is its structure? OH O A) CH,-CH-ċ-H OH B) CH,CH,CH-OH OH O C) CHy OH -CH-C- HO- D) CH,CH, HO-CH,CH-C- H- E) OH 8. Which statement best explains why propanoic acid (M.W. = 74) boils at a much higher temperature than hexane (M.W. = 86)? A) Lower molecular weights tend to boil at higher temperatures. Propanoic acid has weaker intermolecular forces than hexane. Propanoic acid molecules can hydrogen bond with other propanoic molecules but hexane is non-polar and cannot hydrogen bond. D) B) Chains with fewer carbon atoms tend to boil at higher temperatures than ones with more carbon atoms. None of the choices are correct. E) wility: Investigate DFocus
Chapter27: Biomolecules: Lipids
Section27.SE: Something Extra
Problem 42AP: Eleostearic acid, C18H30O2, is a rare fatty acid found in the tung oil used for finishing furniture....
Related questions
Question
100%
Questions 7 and 8
Transcribed Image Text:A Select
Paragraph
Styles
Editing
Voice
Sensitivity
7. Lactic acid, also known as a-hydroxy propionic acid, is an a-hydroxy acid (AHA)
found in some skin care products. What is its structure?
OH
A) CH3-CH-C-H
OH
B) CH;CH,CH-OH
OH
C) CH,-CH-C
-OH
OH
I
D) CH,CH, -C-OH
HO-CH,CH-
H
E)
OH
8. Which statement best explains why propanoic acid (M.W. = 74) boils at a much higher
temperature than hexane (M.W. = 86)?
A)
Lower molecular weights tend to
at higher temperatures.
B)
Propanoic acid has weaker intermolecular forces than hexane.
C)
Propanoic acid molecules can hydrogen bond with other propanoic molecules but
hexane is non-polar and cannot hydrogen bond.
D)
Chains with fewer carbon atoms tend to boil at higher temperatures than ones with
more carbon atoms.
None of the choices are correct.
E)
Accessibility: Investigate
OFocus
prt sc
home
end
Insert
F11
F12
96
&
3
7
8.
P
D
G
K
Expert Solution
Step 1
The answer has been outlined below.
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

