7.00 g of a certain Compound X, known to be made of carbon and hydrogen, and to have a molecular molar mass of 44. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 21.00 g water 11.46 g Use this information to find the molecular formula of X.
7.00 g of a certain Compound X, known to be made of carbon and hydrogen, and to have a molecular molar mass of 44. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 21.00 g water 11.46 g Use this information to find the molecular formula of X.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 9QAP: onsider the balanced chemical equation :math>4Al(s)+3O2(g)2Al2O3(s). at mole ratio would you use to...
Related questions
Question
15
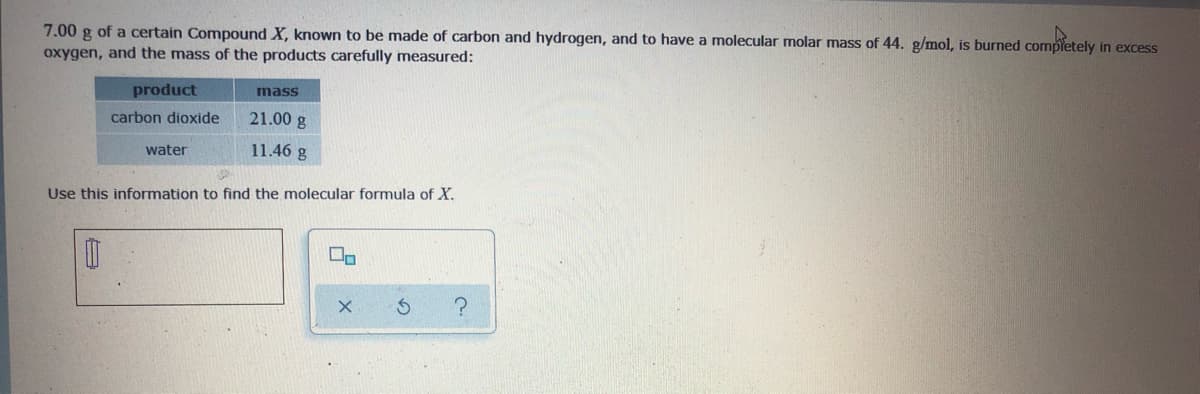
Transcribed Image Text:7.00 g of a certain Compound X, known to be made of carbon and hydrogen, and to have a molecular molar mass of 44. g/mol, is burned completely in excess
oxygen, and the mass of the products carefully measured:
product
mass
carbon dioxide
21.00 g
water
11.46 g
Use this information to find the molecular formula of X.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
