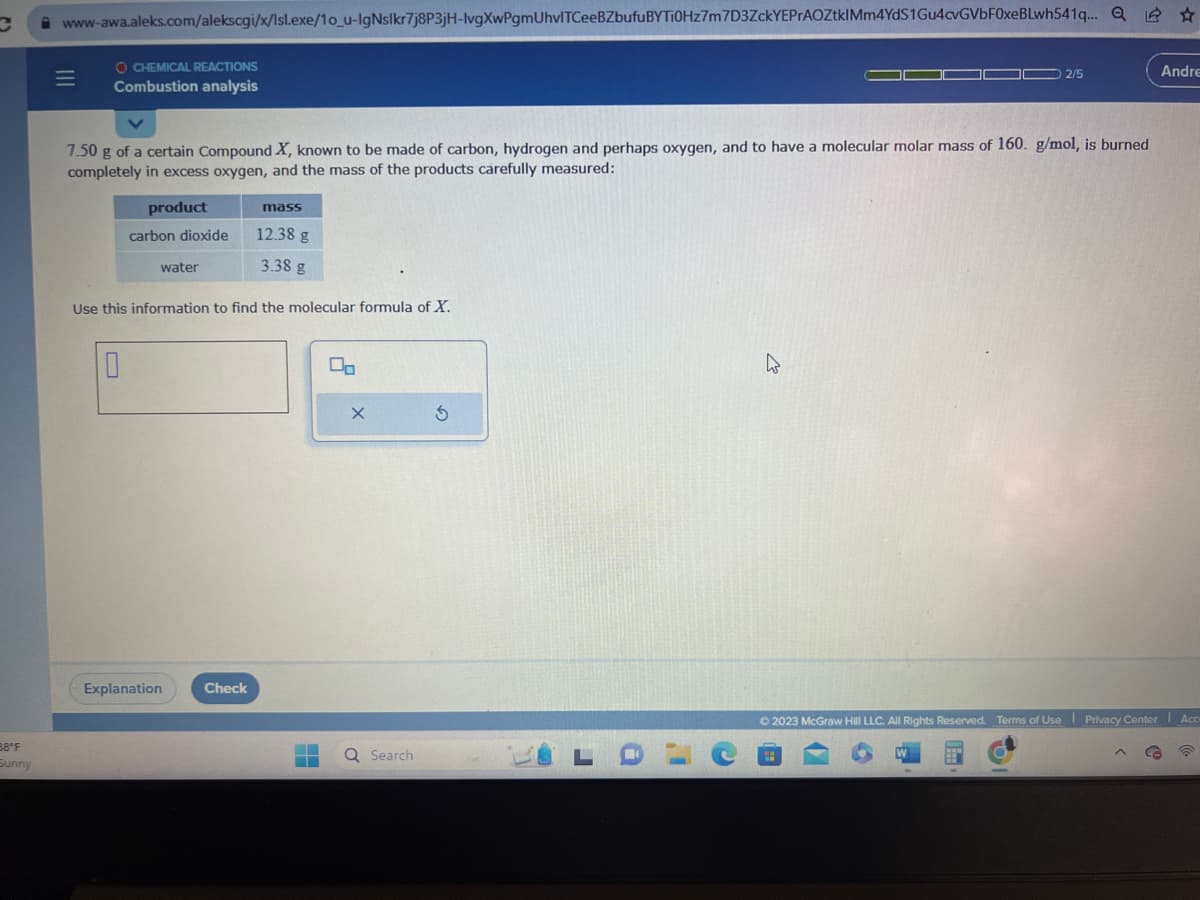
7.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 160. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product carbon dioxide water 10 mass 12.38 g 3.38 g Use this information to find the molecular formula of X.
7.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 160. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product carbon dioxide water 10 mass 12.38 g 3.38 g Use this information to find the molecular formula of X.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Combustions analyzing
Transcribed Image Text:C www-awa.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lvgXwPgmUhvITCeeBZbufu BYTI0Hz7m7D3ZckYEPrAOZtklMm4YdS1Gu4cvGVbF0xeBLwh541q.... Q ✩
38°F
Sunny
=
OCHEMICAL REACTIONS
Combustion analysis
7.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 160. g/mol, is burned
completely in excess oxygen, and the mass of the products carefully measured:
product
carbon dioxide
10
water
Use this information to find the molecular formula of X.
Explanation
mass
Check
12.38 g
3.38 g
T
X
Q Search
S
J
a
I'
C
0 2/5
W
Andre
Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Acc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning