71 Atoms and Elements D. Isotopes and Atomic Mass 1. Nuclear Symbol Protons Neutrons Electrons 107 47Ag 109 47Ag 2. Nuclear Symbol Isotopic Mass Percent Abundance 107 47Ag 106.9 51.84% 109 47Ag 108.9 48.15% (Show calculations here)
71 Atoms and Elements D. Isotopes and Atomic Mass 1. Nuclear Symbol Protons Neutrons Electrons 107 47Ag 109 47Ag 2. Nuclear Symbol Isotopic Mass Percent Abundance 107 47Ag 106.9 51.84% 109 47Ag 108.9 48.15% (Show calculations here)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 37E: An element consists of 1.40% of an isotope with mass 203.973 u, 24.10% of an isotope with mass...
Related questions
Question
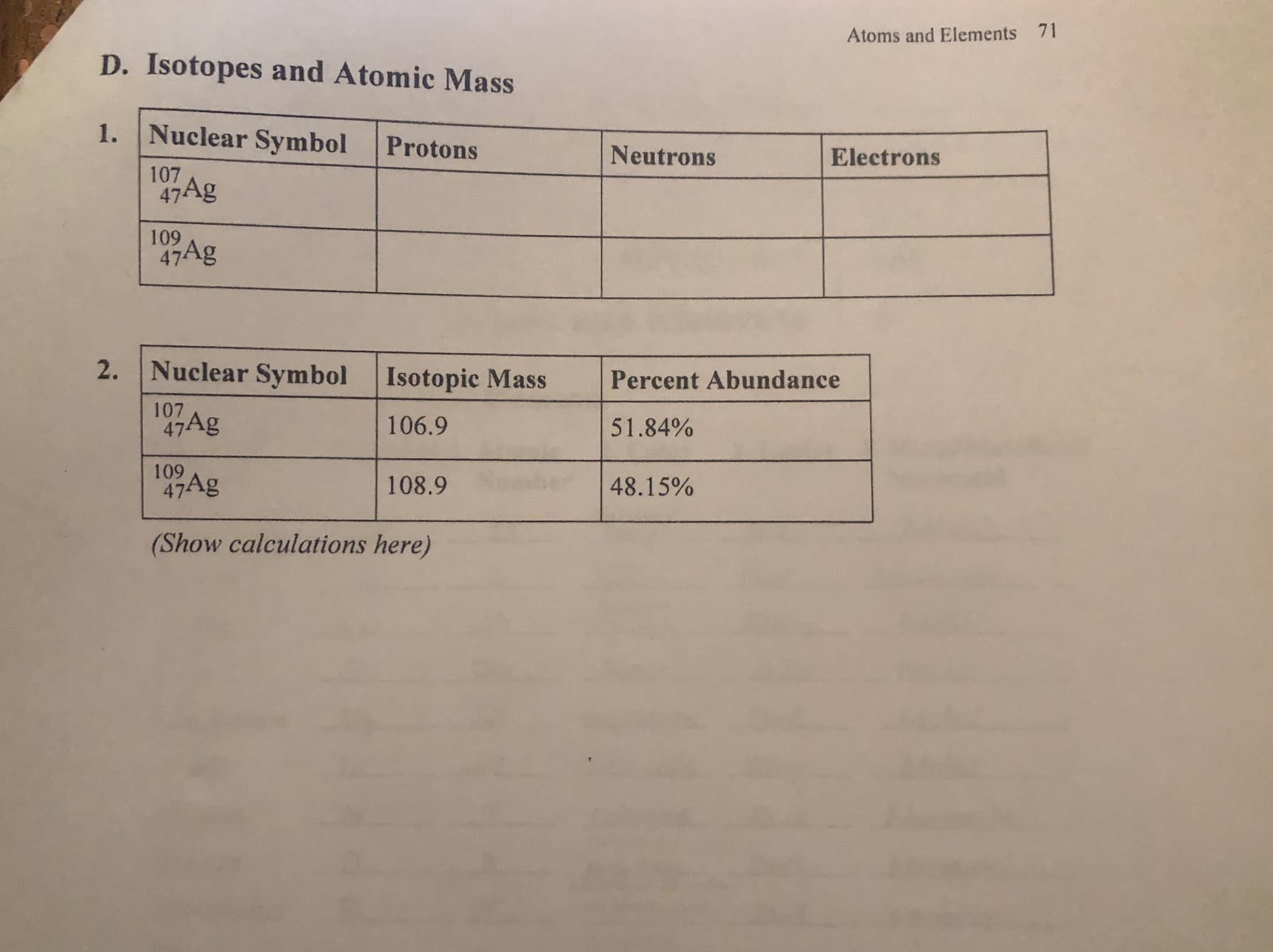
Transcribed Image Text:71
Atoms and Elements
D. Isotopes and Atomic Mass
1. Nuclear Symbol
Protons
Neutrons
Electrons
107
47Ag
109
47Ag
2. Nuclear Symbol
Isotopic Mass
Percent Abundance
107
47Ag
106.9
51.84%
109
47Ag
108.9
48.15%
(Show calculations here)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning